Design Development of the SMARTCLIC®/CLICWISE® Injection Device for Self-Administered Subcutaneous Therapies: Findings from Usability and Human Factor Studies
- PMID: 37199860
- PMCID: PMC10272234
- DOI: 10.1007/s12325-023-02512-2
Design Development of the SMARTCLIC®/CLICWISE® Injection Device for Self-Administered Subcutaneous Therapies: Findings from Usability and Human Factor Studies
Abstract
Introduction: An easy-to-use, multiuse, single-patient, electromechanical autoinjector, the SMARTCLIC®/CLICWISE® injection device, was recently developed to improve the self-administration options available to patients with chronic inflammatory disease treated with biologic agents. An extensive series of studies were conducted to guide the design and development of this device and to ensure its safety and effectiveness.
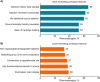
Methods: Participants in two user preference studies and three formative human factor (HF) studies evaluated evolving iterations of the autoinjector device, dose dispenser cartridge, graphical user interface, and informational materials; participants in a summative HF test subsequently assessed the final proposed commercially representative product. In the user preference studies, rheumatologists and patients with chronic inflammatory disease, interviewed online and in-person, provided feedback on the design and functionality of four prototypes. In the HF studies, the safety, effectiveness, and usability of adapted prototypes were assessed under simulated-use conditions by patients with chronic inflammatory disease, caregivers, and healthcare professionals (HCPs). The safety and effectiveness of the final refined device and system were confirmed in a summative HF test by patients and HCPs in simulated-use scenarios.
Results: Rheumatologists (n = 204) and patients (n = 39) interviewed in the two user preference studies provided feedback on the device size, feature ergonomics, and usability that guided prototype development in the subsequent formative HF studies. Observations from patients, caregivers, and HCPs (n = 55) participating in the latter studies yielded additional critical design revisions that culminated in development of the final device and system. Of 106 injection simulations conducted in the summative HF test, all resulted in successful medication delivery, and no potential harms were associated with injection-related use events.
Conclusion: Findings from this research facilitated development of the SmartClic/ClicWise autoinjector device and demonstrated that it could be used safely and effectively by participants representative of the intended-use population of patients, lay caregivers, and HCPs.
Keywords: Anti-TNF; Biologic; Chronic inflammatory disease; Electromechanical autoinjector; Electronic autoinjector device; Human factor; Rheumatoid arthritis; Self-injection; Usability; User preference.
© 2023. The Author(s).
Conflict of interest statement
Kyle Berman, Simon Moss, Barry Holden-Theunissen, Mark Latymer, and Attila Antalfy are employees of Pfizer and hold stock and/or stock options with Pfizer. Nobuhiko Satou and Kenji Okada are employees of PHC Corporation, the manufacturer of the SmartClic/ClicWise device, and hold stock with PHC Corporation.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

