Association of MGMT Promoter Methylation With Survival in Low-grade and Anaplastic Gliomas After Alkylating Chemotherapy
- PMID: 37200021
- PMCID: PMC10196932
- DOI: 10.1001/jamaoncol.2023.0990
Association of MGMT Promoter Methylation With Survival in Low-grade and Anaplastic Gliomas After Alkylating Chemotherapy
Erratum in
-
Errors in Figure 3.JAMA Oncol. 2023 Jul 1;9(7):1009. doi: 10.1001/jamaoncol.2023.2581. JAMA Oncol. 2023. PMID: 37470838 Free PMC article. No abstract available.
Abstract
Importance: O6-methylguanine-DNA methyltransferase (MGMT [OMIM 156569]) promoter methylation (mMGMT) is predictive of response to alkylating chemotherapy for glioblastomas and is routinely used to guide treatment decisions. However, the utility of MGMT promoter status for low-grade and anaplastic gliomas remains unclear due to molecular heterogeneity and the lack of sufficiently large data sets.
Objective: To evaluate the association of mMGMT for low-grade and anaplastic gliomas with chemotherapy response.
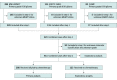
Design, setting, and participants: This cohort study aggregated grade II and III primary glioma data from 3 prospective cohort studies with patient data collected from August 13, 1995, to August 3, 2022, comprising 411 patients: MSK-IMPACT, EORTC (European Organization of Research and Treatment of Cancer) 26951, and Columbia University. Statistical analysis was performed from April 2022 to January 2023.
Exposure: MGMT promoter methylation status.
Main outcomes and measures: Multivariable Cox proportional hazards regression modeling was used to assess the association of mMGMT status with progression-free survival (PFS) and overall survival (OS) after adjusting for age, sex, molecular class, grade, chemotherapy, and radiotherapy. Subgroups were stratified by treatment status and World Health Organization 2016 molecular classification.
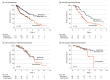
Results: A total of 411 patients (mean [SD] age, 44.1 [14.5] years; 283 men [58%]) met the inclusion criteria, 288 of whom received alkylating chemotherapy. MGMT promoter methylation was observed in 42% of isocitrate dehydrogenase (IDH)-wild-type gliomas (56 of 135), 53% of IDH-mutant and non-codeleted gliomas (79 of 149), and 74% of IDH-mutant and 1p/19q-codeleted gliomas (94 of 127). Among patients who received chemotherapy, mMGMT was associated with improved PFS (median, 68 months [95% CI, 54-132 months] vs 30 months [95% CI, 15-54 months]; log-rank P < .001; adjusted hazard ratio [aHR] for unmethylated MGMT, 1.95 [95% CI, 1.39-2.75]; P < .001) and OS (median, 137 months [95% CI, 104 months to not reached] vs 61 months [95% CI, 47-97 months]; log-rank P < .001; aHR, 1.65 [95% CI, 1.11-2.46]; P = .01). After adjusting for clinical factors, MGMT promoter status was associated with chemotherapy response in IDH-wild-type gliomas (aHR for PFS, 2.15 [95% CI, 1.26-3.66]; P = .005; aHR for OS, 1.69 [95% CI, 0.98-2.91]; P = .06) and IDH-mutant and codeleted gliomas (aHR for PFS, 2.99 [95% CI, 1.44-6.21]; P = .003; aHR for OS, 4.21 [95% CI, 1.25-14.2]; P = .02), but not IDH-mutant and non-codeleted gliomas (aHR for PFS, 1.19 [95% CI, 0.67-2.12]; P = .56; aHR for OS, 1.07 [95% CI, 0.54-2.12]; P = .85). Among patients who did not receive chemotherapy, mMGMT status was not associated with PFS or OS.
Conclusions and relevance: This study suggests that mMGMT is associated with response to alkylating chemotherapy for low-grade and anaplastic gliomas and may be considered as a stratification factor in future clinical trials of patients with IDH-wild-type and IDH-mutant and codeleted tumors.
Conflict of interest statement
Figures

Comment in
-
MGMT Methylation Status in Grades 2 and 3 Gliomas Is Important, but Is It Prognostic?JAMA Oncol. 2023 Jul 1;9(7):928-929. doi: 10.1001/jamaoncol.2023.0759. JAMA Oncol. 2023. PMID: 37200043 No abstract available.
-
MGMT Promoter Methylation and Chemotherapy Outcomes in Low-Grade and Anaplastic Gliomas.JAMA Oncol. 2023 Dec 1;9(12):1734-1735. doi: 10.1001/jamaoncol.2023.4736. JAMA Oncol. 2023. PMID: 37856142 No abstract available.
References
-
- Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707-715. doi:10.1016/S1470-2045(12)70164-X - DOI - PubMed
-
- Malmström A, Grønberg BH, Marosi C, et al. ; Nordic Clinical Brain Tumour Study Group (NCBTSG) . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916-926. doi:10.1016/S1470-2045(12)70265-6 - DOI - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. Version 1.2021. National Comprehensive Cancer Network; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

