Panitumumab Plus Trifluridine-Tipiracil as Anti-Epidermal Growth Factor Receptor Rechallenge Therapy for Refractory RAS Wild-Type Metastatic Colorectal Cancer: A Phase 2 Randomized Clinical Trial
- PMID: 37200022
- PMCID: PMC10196928
- DOI: 10.1001/jamaoncol.2023.0655
Panitumumab Plus Trifluridine-Tipiracil as Anti-Epidermal Growth Factor Receptor Rechallenge Therapy for Refractory RAS Wild-Type Metastatic Colorectal Cancer: A Phase 2 Randomized Clinical Trial
Erratum in
-
Error in Affiliations.JAMA Oncol. 2024 Apr 1;10(4):541. doi: 10.1001/jamaoncol.2024.0068. JAMA Oncol. 2024. PMID: 38386352 Free PMC article. No abstract available.
Abstract
Importance: Current third-line therapies for patients with metastatic colorectal cancer (MCRC) have limited efficacy. Rechallenge with epidermal growth factor receptor (EGFR) inhibitors for RAS wild-type (WT) MCRC may be valuable for these patients.
Objective: To compare the anti-EGFR monoclonal antibody panitumumab plus standard-of-care trifluridine-tipiracil with trifluridine-tipiracil alone as third-line therapy for RAS WT MCRC.
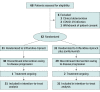
Design, setting, and participants: This phase 2 randomized clinical trial (RCT) was conducted in 7 Italian centers from June 2019 to April 2022. Patients with refractory RAS WT MCRC who had a partial or complete response to first-line chemotherapy plus an anti-EGFR monoclonal antibody and an anti-EGFR drug-free interval of 4 or more months during second-line therapy were included.
Interventions: Patients were randomized 1:1 to receive panitumumab plus trifluridine-tipiracil or trifluridine-tipiracil alone.
Main outcomes and measures: The primary end point was progression-free survival (PFS). Circulating tumor DNA (ctDNA) extended sequence variation analysis was performed in a subgroup of patients.
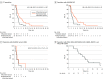
Results: Of 62 included patients, 31 received panitumumab plus trifluridine-tipiracil (19 [61.3%] male; median age, 65 years [range, 39-81 years]) and 31 received trifluridine-tipiracil alone (17 [54.8%] male; median age, 66 years [range, 32-82 years]). The primary end point was met. Median PFS was 4.0 months (95% CI, 2.8-5.3 months) in the panitumumab plus trifluridine-tipiracil arm vs 2.5 months (95% CI, 1.4-3.6 months) in the trifluridine-tipiracil only (hazard ratio [HR], 0.48; 95% CI, 0.28-0.82; P = .007). Pretreatment plasma RAS/BRAF WT ctDNA identified patients obtaining prolonged clinical benefit with panitumumab plus trifluridine-tipiracil compared with trifluridine-tipiracil, with PFS rates at 6 months of 38.5% vs 13.0% and at 12 months of 15.4% vs 0%. A ctDNA liquid-biopsy extended mutation analysis by FoundationOne Liquid CDx (profiling 324 genes) was performed in a subgroup of patients with baseline plasma RAS/BRAF WT ctDNA; in 15 of 23 patients (65.2%) whose tumors were WT for KRAS, NRAS, BRAFV600E, EGFR, ERBB2, MAP2K1, and PIK3CA, median PFS was 6.4 months (95% CI, 3.7-9.2 months). Within this group of 15 patients, 2 (13.3%) had partial response, 11 (73.3%) had stable disease, and 2 (13.3%) had disease progression as best response.
Conclusions and relevance: In this RCT, third-line treatment with the anti-EGFR monoclonal antibody panitumumab plus the standard-of-care trifluridine-tipiracil resulted in improved PFS compared with treatment with trifluridine-tipiracil alone among patients with refractory RAS WT MCRC. The findings support the clinical utility of liquid biopsy-guided anti-EGFR rechallenge therapy for refractory RAS WT MCRC.
Trial registration: ClinicalTrials.gov Identifier: NCT05468892.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

