Myofiber Type Shift in Extraocular Muscles in Amyotrophic Lateral Sclerosis
- PMID: 37200039
- PMCID: PMC10207955
- DOI: 10.1167/iovs.64.5.15
Myofiber Type Shift in Extraocular Muscles in Amyotrophic Lateral Sclerosis
Abstract
Purpose: To investigate changes in myofiber composition in the global layer (GL) and orbital layer (OL) of extraocular muscles (EOMs) from terminal amyotrophic lateral sclerosis (ALS) donors.
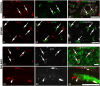
Methods: Medial recti muscles collected postmortem from spinal-onset ALS, bulbar-onset ALS, and healthy control donors were processed for immunofluorescence with antibodies against myosin heavy chain (MyHC) IIa, MyHCI, MyHCeom, laminin, neurofilaments, synaptophysin, acetylcholine receptor γ-subunit, and α-bungarotoxin.
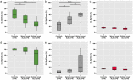
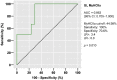
Results: The proportion of myofibers containing MyHCIIa was significantly smaller and MyHCeom was significantly larger in the GL of spinal-onset ALS and bulbar-onset ALS donors compared to control donors. Changes in the GL were more prominent in the bulbar-onset ALS donors, with a significantly larger proportion of myofibers containing MyHCeom being present compared to spinal-onset ALS donors. There were no significant differences in the myofiber composition in the OL. In the spinal-onset ALS donors, the proportions of myofibers containing MyHCIIa in the GL and MyHCeom in the OL were significantly correlated with the disease duration. Neurofilament and synaptophysin were present at motor endplates of myofibers containing MyHCeom in ALS donors.
Conclusions: The EOMs of terminal ALS donors displayed changes in the fast-type myofiber composition in the GL, with a more pronounced alteration in bulbar-onset ALS donors. Our results align with the worse prognosis and subclinical changes in eye movement function previously observed in bulbar-onset ALS patients and suggest that the myofibers in the OL might be more resistant to the pathological process in ALS.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
Impact of Amyotrophic Lateral Sclerosis on Slow Tonic Myofiber Composition in Human Extraocular Muscles.Invest Ophthalmol Vis Sci. 2017 Jul 1;58(9):3708-3715. doi: 10.1167/iovs.17-22098. Invest Ophthalmol Vis Sci. 2017. PMID: 28738414
-
A Novel Type of Multiterminal Motor Endplate in Human Extraocular Muscles.Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):539-548. doi: 10.1167/iovs.17-22554. Invest Ophthalmol Vis Sci. 2018. PMID: 29372252
-
Different impact of ALS on laminin isoforms in human extraocular muscles versus limb muscles.Invest Ophthalmol Vis Sci. 2011 Jul 1;52(7):4842-52. doi: 10.1167/iovs.10-7132. Invest Ophthalmol Vis Sci. 2011. PMID: 21372009
-
Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis.Acta Neuropathol. 2017 Jun;133(6):863-885. doi: 10.1007/s00401-017-1708-8. Epub 2017 Apr 13. Acta Neuropathol. 2017. PMID: 28409282 Free PMC article. Review.
-
Amyotrophic Lateral Sclerosis Regional Variants (Brachial Amyotrophic Diplegia, Leg Amyotrophic Diplegia, and Isolated Bulbar Amyotrophic Lateral Sclerosis).Neurol Clin. 2015 Nov;33(4):775-85. doi: 10.1016/j.ncl.2015.07.003. Epub 2015 Sep 8. Neurol Clin. 2015. PMID: 26515621 Free PMC article. Review.
Cited by
-
Extreme Tolerance of Extraocular Muscles to Diseases and Aging: Why and How?Int J Mol Sci. 2024 May 3;25(9):4985. doi: 10.3390/ijms25094985. Int J Mol Sci. 2024. PMID: 38732204 Free PMC article. Review.
-
Obscurin Maintains Myofiber Identity in Extraocular Muscles.Invest Ophthalmol Vis Sci. 2024 Feb 1;65(2):19. doi: 10.1167/iovs.65.2.19. Invest Ophthalmol Vis Sci. 2024. PMID: 38334702 Free PMC article.
-
Exploring amyotrophic lateral sclerosis through the visual system: A systematic review.Eur J Neurol. 2024 Dec;31(12):e16475. doi: 10.1111/ene.16475. Epub 2024 Sep 20. Eur J Neurol. 2024. PMID: 39302063 Free PMC article.
References
-
- Swinnen B, Robberecht W.. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014; 10: 661–670. - PubMed
-
- Westeneng H-J, Debray TPA, Visser AE, et al. .. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018; 17: 423–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

