The cell envelope of Mycobacterium abscessus and its role in pathogenesis
- PMID: 37200238
- PMCID: PMC10194924
- DOI: 10.1371/journal.ppat.1011318
The cell envelope of Mycobacterium abscessus and its role in pathogenesis
Abstract
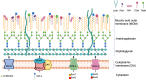
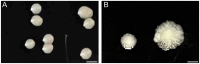
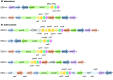
Mycobacterium abscessus is a nontuberculosis mycobacterium (NTM) that has shown an exponential rise in its ability to cause disease. Due to its ubiquitous presence in the environment, M. abscessus is widely implicated in secondary exacerbations of many nosocomial infections and genetic respiratory disorders, such as cystic fibrosis (CF). Contrary to other rapidly growing NTMs, the cell envelope of M. abscessus harbors several prominent features and undergoes modifications that are responsible for its pathogenesis. Compositional changes of the mycobacterial outer membrane (MOM) significantly decrease the presence of glycopeptidolipids (GPLs) and enable the transition from a colonizing, smooth morphotype into a virulent, rough morphotype. The GPLs are transported to the MOM by the Mycobacterial membrane proteins Large (MmpL), which further act as drug efflux pumps and confer antibiotic resistance. Lastly, M. abscessus possesses 2 type VII secretion systems (T7SS): ESX-3 and ESX-4, both of which have recently been implicated in host-pathogen interactions and virulence. This review summarizes the current knowledge of M. abscessus pathogenesis and highlights the clinically relevant association between the structure and functions of its cell envelope.
Copyright: © 2023 Parmar, Tocheva. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

