Recapitulating the Lateral Organization of Membrane Receptors at the Nanoscale
- PMID: 37200265
- PMCID: PMC10278181
- DOI: 10.1021/acsnano.3c00683
Recapitulating the Lateral Organization of Membrane Receptors at the Nanoscale
Abstract
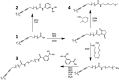
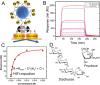
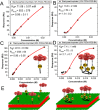
Many cell membrane functions emerge from the lateral presentation of membrane receptors. The link between the nanoscale organization of the receptors and ligand binding remains, however, mostly unclear. In this work, we applied surface molecular imprinting and utilized the phase behavior of lipid bilayers to create platforms that recapitulate the lateral organization of membrane receptors at the nanoscale. We used liposomes decorated with amphiphilic boronic acids that commonly serve as synthetic saccharide receptors and generated three lateral modes of receptor presentation─random distribution, nanoclustering, and receptor crowding─and studied their interaction with saccharides. In comparison to liposomes with randomly dispersed receptors, surface-imprinted liposomes resulted in more than a 5-fold increase in avidity. Quantifying the binding affinity and cooperativity proved that the boost was mediated by the formation of the nanoclusters rather than a local increase in the receptor concentration. In contrast, receptor crowding, despite the presence of increased local receptor concentrations, prevented multivalent oligosaccharide binding due to steric effects. The findings demonstrate the significance of nanometric aspects of receptor presentation and generation of multivalent ligands including artificial lectins for the sensitive and specific detection of glycans.
Keywords: membrane receptor; multivalent interaction; nanoclusters; receptor crowding; surface molecular imprinting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Avidity and surface mobility in multivalent ligand-receptor binding.Nanoscale. 2021 Aug 7;13(29):12602-12612. doi: 10.1039/d1nr02083h. Epub 2021 Jul 14. Nanoscale. 2021. PMID: 34259699 Free PMC article.
-
Synthesis and Applications of Boronate Affinity Materials: From Class Selectivity to Biomimetic Specificity.Acc Chem Res. 2017 Sep 19;50(9):2185-2193. doi: 10.1021/acs.accounts.7b00179. Epub 2017 Aug 29. Acc Chem Res. 2017. PMID: 28849912
-
Aromaticity/Bulkiness of Surface Ligands to Promote the Interaction of Anionic Amphiphilic Gold Nanoparticles with Lipid Bilayers.Langmuir. 2016 Feb 16;32(6):1601-10. doi: 10.1021/acs.langmuir.6b00035. Epub 2016 Feb 3. Langmuir. 2016. PMID: 26794292
-
Selective sensing of saccharides using simple boronic acids and their aggregates.Chem Soc Rev. 2013 Oct 21;42(20):8032-48. doi: 10.1039/c3cs60148j. Chem Soc Rev. 2013. PMID: 23860576 Review.
-
Significance of Receptor Mobility in Multivalent Binding on Lipid Membranes.Angew Chem Int Ed Engl. 2022 Mar 21;61(13):e202114167. doi: 10.1002/anie.202114167. Epub 2022 Jan 28. Angew Chem Int Ed Engl. 2022. PMID: 34982497 Free PMC article. Review.
References
-
- Wolf A. A.; Jobling M. G.; Wimer-Mackin S.; Ferguson-Maltzman M.; Madara J. L.; Holmes R. K.; Lencer W. I. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J. Cell Biol. 1998, 141 (4), 917–927. 10.1083/jcb.141.4.917. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

