Presence and activity of Fibrinogen like protein 2 in platelets
- PMID: 37200306
- PMCID: PMC10194929
- DOI: 10.1371/journal.pone.0285735
Presence and activity of Fibrinogen like protein 2 in platelets
Abstract
Background: Fibrinogen-like protein 2 (FGL2) is a serine protease capable of converting prothrombin into thrombin (i.e., prothrombinase-like activity) while bypassing the classic coagulation cascade. It has been reported to be expressed by mononuclear blood cells and endothelial cells. There are multiple reports that FGL2 supports tumor development and metastasis. However, in the blood, the origin and functional significance of FGL2 has not been established.
Objective: To determine if FGL2, a malignancy related enzyme, is present in platelets.
Methods: Peripheral blood samples were collected in K2 EDTA tubes. Blood cells and platelets were separated and thoroughly washed to produce plasma-free samples. Procoagulant activity was measured in the cell lysates using a thrombin generation test or an adjusted prothrombin time (PT) test in plasma deficient of factor X. The findings were further supported by confocal microscopy, immunoprecipitation, flow cytometry, enzyme-linked immunosorbent assays and specific inhibition assays.
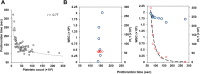
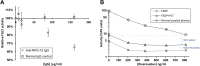
Results: FGL2 protein was readily detected in platelets. Also, despite being expressed by lymphocytes, FGL2 prothrombinase-like activity was solely detected in platelet samples, but not in white blood cell samples. Quiescent platelets were shown to contain the FGL2 protein in an active form. Upon activation, platelets secreted the active FGL2 into the milieu.
Conclusions: Active FGL2 is found in platelets. This suggests another role for the involvement of platelets in malignancies.
Copyright: © 2023 Cherny et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The FGL2 prothrombinase contributes to the pathological process of experimental pulmonary hypertension.J Appl Physiol (1985). 2019 Dec 1;127(6):1677-1687. doi: 10.1152/japplphysiol.00396.2019. Epub 2019 Oct 3. J Appl Physiol (1985). 2019. PMID: 31580221
-
Kinetic analysis of a unique direct prothrombinase, fgl2, and identification of a serine residue critical for the prothrombinase activity.J Immunol. 2002 May 15;168(10):5170-7. doi: 10.4049/jimmunol.168.10.5170. J Immunol. 2002. PMID: 11994472
-
Fibrinogen-related protein, FGL2, of hamster cauda epididymal fluid: Purification, kinetic analysis of its prothrombinase activity, and its role in segregation of nonviable spermatozoa.Mol Reprod Dev. 2020 Dec;87(12):1206-1218. doi: 10.1002/mrd.23438. Epub 2020 Nov 20. Mol Reprod Dev. 2020. PMID: 33216420 Free PMC article.
-
Role of fibrinogen-like 2 (FGL2) proteins in implantation: Potential implications and mechanism.Gene. 2025 Apr 20;946:149284. doi: 10.1016/j.gene.2025.149284. Epub 2025 Jan 28. Gene. 2025. PMID: 39884406 Review.
-
The Duality of Fgl2 - Secreted Immune Checkpoint Regulator Versus Membrane-Associated Procoagulant: Therapeutic Potential and Implications.Int Rev Immunol. 2016 Jul 3;35(4):325-339. doi: 10.3109/08830185.2014.956360. Epub 2014 Sep 26. Int Rev Immunol. 2016. PMID: 25259408 Free PMC article. Review.
References
-
- Marazzi S, Blum S, Hartmann R, Gundersen D, Schreyer M, Argraves S, et al.. Characterization of human fibroleukin, a fibrinogen-like protein secreted by T lymphocytes. J Immunol. 1998;161(1):138–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

