Melatonin alleviates lung injury in H1N1-infected mice by mast cell inactivation and cytokine storm suppression
- PMID: 37200384
- PMCID: PMC10249807
- DOI: 10.1371/journal.ppat.1011406
Melatonin alleviates lung injury in H1N1-infected mice by mast cell inactivation and cytokine storm suppression
Abstract
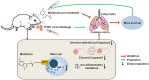
Influenza A virus (IAV) H1N1 infection is a constant threat to human health and it remains so due to the lack of an effective treatment. Since melatonin is a potent antioxidant and anti-inflammatory molecule with anti-viral action, in the present study we used melatonin to protect against H1N1 infection under in vitro and in vivo conditions. The death rate of the H1N1-infected mice was negatively associated with the nose and lung tissue local melatonin levels but not with serum melatonin concentrations. The H1N1-infected AANAT-/- melatonin-deficient mice had a significantly higher death rate than that of the WT mice and melatonin administration significantly reduced the death rate. All evidence confirmed the protective effects of melatonin against H1N1 infection. Further study identified that the mast cells were the primary targets of melatonin action, i.e., melatonin suppresses the mast cell activation caused by H1N1 infection. The molecular mechanisms involved melatonin down-regulation of gene expression for the HIF-1 pathway and inhibition of proinflammatory cytokine release from mast cells; this resulted in a reduction in the migration and activation of the macrophages and neutrophils in the lung tissue. This pathway was mediated by melatonin receptor 2 (MT2) since the MT2 specific antagonist 4P-PDOT significantly blocked the effects of melatonin on mast cell activation. Via targeting mast cells, melatonin suppressed apoptosis of alveolar epithelial cells and the lung injury caused by H1N1 infection. The findings provide a novel mechanism to protect against the H1N1-induced pulmonary injury, which may better facilitate the progress of new strategies to fight H1N1 infection or other IAV viral infections.
Copyright: © 2023 Huo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
PI3K/AKT-mediated autophagy inhibition facilitates mast cell activation to enhance severe inflammatory lung injury in influenza A virus- and secondary Staphylococcus aureus-infected mice.Antiviral Res. 2023 Jan;209:105502. doi: 10.1016/j.antiviral.2022.105502. Epub 2022 Dec 20. Antiviral Res. 2023. PMID: 36549394
-
Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling.J Immunol. 2013 May 1;190(9):4676-84. doi: 10.4049/jimmunol.1202096. Epub 2013 Mar 22. J Immunol. 2013. PMID: 23526820 Free PMC article.
-
H1N1 influenza virus dose dependent induction of dysregulated innate immune responses and STAT1/3 activation are associated with pulmonary immunopathological damage.Virulence. 2022 Dec;13(1):1558-1572. doi: 10.1080/21505594.2022.2120951. Virulence. 2022. PMID: 36082929 Free PMC article.
-
Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection.JCI Insight. 2018 Dec 20;3(24):e99022. doi: 10.1172/jci.insight.99022. JCI Insight. 2018. PMID: 30568032 Free PMC article.
-
Total alkaloids from Alstonia scholaris inhibit influenza a virus replication and lung immunopathology by regulating the innate immune response.Phytomedicine. 2020 Oct;77:153272. doi: 10.1016/j.phymed.2020.153272. Epub 2020 Jun 29. Phytomedicine. 2020. PMID: 32702592
Cited by
-
Melatonin alleviates sepsis-induced acute lung injury by inhibiting necroptosis via reducing circulating mtDNA release.Mol Med. 2025 May 7;31(1):176. doi: 10.1186/s10020-025-01228-z. Mol Med. 2025. PMID: 40335920 Free PMC article.
-
Melatonin, BAG-1 and cortisol circadian interactions in tumor pathogenesis and patterned immune responses.Explor Target Antitumor Ther. 2023;4(5):962-993. doi: 10.37349/etat.2023.00176. Epub 2023 Oct 25. Explor Target Antitumor Ther. 2023. PMID: 37970210 Free PMC article. Review.
-
Melatonin Interplay in Physiology and Disease-The Fountain of Eternal Youth Revisited.Biomolecules. 2025 May 8;15(5):682. doi: 10.3390/biom15050682. Biomolecules. 2025. PMID: 40427575 Free PMC article. Review.
-
UiO-66 nanoparticles combat influenza A virus in mice by activating the RIG-I-like receptor signaling pathway.J Nanobiotechnology. 2024 Mar 9;22(1):99. doi: 10.1186/s12951-024-02358-y. J Nanobiotechnology. 2024. PMID: 38461229 Free PMC article.
-
Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective.Life (Basel). 2025 Jan 16;15(1):113. doi: 10.3390/life15010113. Life (Basel). 2025. PMID: 39860053 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

