Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study
- PMID: 37201046
- PMCID: PMC10186513
- DOI: 10.21037/jgo-23-108
Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study
Abstract
Background: The most effective treatment with immune checkpoint inhibitors (ICIs) is limited to the microsatellite instability high (MSI-H) subgroup of advanced colorectal cancer. ICIs are completely ineffective in microsatellite stabilized (MSS) patients with advanced colorectal cancer. Fruquintinib, a tyrosine kinase inhibitor (TKI) domestically made in China that specifically inhibits vascular endothelial growth factor receptors, is used to treat refractory metastatic colorectal cancer (mCRC). Researches showed that anti-angiogenic therapy combined with immunotherapy induces a long-lasting antitumor immune response. Here, we aimed to evaluate antitumor efficacy and safety of fruquintinib with anti-programmed death-1 (PD-1) antibody toripalimab in Chinese patients with non-MSI-H/mismatch repair proficient (pMMR) mCRC.
Methods: This was a single-arm, single-center, prospective, phase II clinical trial. A total of 19 MSS patients with refractory or advanced mCRC were enrolled They received fruquintinib (5 mg, orally, once daily for 3 weeks followed by 1 week off in 4-week cycles) and toripalimab (240 mg, intravenously administered on day 1 once every 3 weeks) until disease progression or unacceptable toxicity. The objective response rate (ORR), progression-free survival (PFS), overall survival (OS), 1-year PFS rate, disease control rate (DCR), and toxicity were reviewed and evaluated. The Cox regression model was used to analyze the influence on OS and PFS.
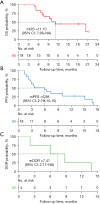
Results: Among the 19 patients, the median age was 52 years (range, 30-71 years); 4 patients (21.05%) achieved partial response, 10 patients (52.63%) experienced stable disease, and 4 patients (21.05%) experienced progressive disease. The ORR was 21.05%. The median PFS and OS were 5.98 months and 11.10 months, respectively. Patients with peritoneal metastasis received greater benefit from combination therapy, with a longer PFS (P=0.043) in the univariate analysis. The most common treatment-related adverse reactions were fatigue (57.89%), hepatic dysfunction (42.11%) and hypertension (36.84%). No serious adverse effects or adverse effect-related deaths were reported.
Conclusions: Our study provides evidence supporting fruquintinib combined with an anti-PD-1 monoclonal antibody have the better effect than fruquintinib alone in the third-line setting for Chinese patients with MSS advanced colorectal cancer. Primary lesion excision and peritoneal metastasis were independent prognostic factors of PFS. Further well-designed, prospective, large-scale studies are needed to validate this outcome.
Keywords: Fruquintinib; anti-programmed death-1 (PD-1) inhibitors; metastatic colorectal cancer (mCRC); non-microsatellite instability high/mismatch repair proficient (non-MSI-H/pMMR).
2023 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-108/coif). All authors report that this study was sponsored by HUTCHMED Limited and Shanghai Junshi Biosciences. The authors have no other conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
