A single-center retrospective analysis of the efficacy and safety of a modified regimen of irinotecan plus S-1 (IRIS) with molecular targeting agents as second-line chemotherapy in Japanese patients with recurrent or nonresectable colorectal cancer
- PMID: 37201062
- PMCID: PMC10186548
- DOI: 10.21037/jgo-22-899
A single-center retrospective analysis of the efficacy and safety of a modified regimen of irinotecan plus S-1 (IRIS) with molecular targeting agents as second-line chemotherapy in Japanese patients with recurrent or nonresectable colorectal cancer
Abstract
Background: As the second-line chemotherapy for stage IV recurrent or nonresectable colorectal cancer, our hospital started a modified treatment regimen comprising of irinotecan plus S-1 (IRIS) [tegafur/gimeracil/oteracil (S-1)] plus molecular targeting agents (MTAs), i.e., an epidermal growth factor receptor (EGFR) inhibitor such as panitumumab (P-mab) or cetuximab (C-mab) or vascular endothelial growth factor (VEGF) inhibitor such as bevacizumab (B-mab) since October 2012. The purpose of this study is to evaluate the efficacy and safety of this modified regimen.
Methods: This retrospective study included 41 patients with advanced recurrent colorectal cancer at our hospital whom at least 3 courses of chemotherapy were conducted from January 2015 to December 2021. Based on the location of the primary tumor, patients were classified into two group (right-sided group, proximal to the splenic curve, and left-sided, distal to the splenic curve). We assessed archived data on RAS and BRAF status and UGT1A1 polymorphisms and use of the VEGF inhibitor bevacizumab (B-mab) and the EGFR inhibitors panitumumab (P-mab) and cetuximab (C-mab). In addition, progression-free survival rate (36M-PFS) and the overall survival rate (36M-OS) were calculated. Furthermore, the respective median survival time (MST), the median number of treatment courses; the objective response rate (ORR) and clinical benefit rate (CBR) and the incidence of adverse events (AEs) were assessed as well.
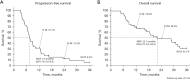
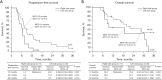
Results: There were 11 patients (26.8%) in the right-sided group, and 30 patients (73.2%) in the left-sided group. There were 19 patients with RAS wild type (46.3%) (1 in the right sided group and 18 in the left sided group). P-mab was used for 16 of these patients (84.2%), C-mab for 2 (10.5%), and B-mab for 1 (5.3%); the remaining 22 patients (53.7%). Ten patients in the right group and 12 patients in the left group were a mutated type and received B-mab. BRAF testing was performed in 17 patients (41.5%); as more than 50% of patients (58.5%) were included before the assay's introduction. Five patients in the right-sided group and 12 patients in the left-sided group had wild type. There was no mutated type. UGT1A1 polymorphism was tested in 16/41 patients: Eight were wild type (8/41 patients, 19.5%) and 8, mutated type. Regarding the *6/*28 double heterozygous type, there was only 1 patient in the right-sided group and the remaining 7 patients were in the left-sided group. The total number of chemotherapy courses was 299, and the median number, 6.0 (range, 3-20). PFS, OS, and MST were as follows: 36M-PFS (total/Rt/Lt), 6.2%/0.0%/8.5% (MST; 7.6/6.3/8.9 months); and 36M-OS (total/Rt/Lt), 32.1%/0.0%/44.0% (MST; 22.1/18.8/28.6 months). The ORR and CBR were 24.4% and 75.6%, respectively. The majority of AEs were grades 1 or 2 and were improved with conservative treatment. Grade 3 leukopenia was observed in 2 cases (4.9%), neutropenia in 4 cases (9.8%), and malaise/nausea/diarrhea/perforation in 1 case each (2.4%). Grade 3 leukopenia (2 patients) and neutropenia (3 patients) were more commonly observed in the left-sided group. Diarrhea and perforation were also common in the left-sided group.
Conclusions: This second-line modified IRIS regimen with MTAs is safe and effective and results in good PFS and OS.
Keywords: Colorectal cancer; chemotherapy; irinotecan plus S-1 (IRIS); molecular targeting therapy; recurrent/non-resectable cancer.
2023 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-899/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials.Ann Oncol. 2017 Aug 1;28(8):1713-1729. doi: 10.1093/annonc/mdx175. Ann Oncol. 2017. PMID: 28407110 Free PMC article.
-
FOLFOXIRI-Bevacizumab or FOLFOX-Panitumumab in Patients with Left-Sided RAS/BRAF Wild-Type Metastatic Colorectal Cancer: A Propensity Score-Based Analysis.Oncologist. 2021 Apr;26(4):302-309. doi: 10.1002/onco.13642. Epub 2021 Jan 2. Oncologist. 2021. PMID: 33336844 Free PMC article.
-
Efficacy of modified bevacizumab-XELOX therapy in Japanese patients with stage IV recurrent or non-resectable colorectal cancer.J Gastrointest Oncol. 2021 Apr;12(2):527-534. doi: 10.21037/jgo-20-350. J Gastrointest Oncol. 2021. PMID: 34012646 Free PMC article.
-
Primary tumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: a systematic review and pooled analysis of randomised trials.Eur J Cancer. 2023 May;184:106-116. doi: 10.1016/j.ejca.2023.02.006. Epub 2023 Feb 17. Eur J Cancer. 2023. PMID: 36913832
Cited by
-
Combination of potassium oxonate with anti-PD-1 for the treatment of colorectal cancer.Front Oncol. 2025 Feb 7;15:1528004. doi: 10.3389/fonc.2025.1528004. eCollection 2025. Front Oncol. 2025. PMID: 39990679 Free PMC article.
References
-
- Modest DP, Ricard I, Heinemann V, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 2016;27:1746-53. 10.1093/annonc/mdw261 - DOI - PMC - PubMed
-
- Van Cutsem E, Huijberts S, Grothey A, et al. Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From the Phase III BEACON Colorectal Cancer Study. J Clin Oncol 2019;37:1460-9. 10.1200/JCO.18.02459 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
