Performance of a blood-based RNA signature for gemcitabine-based treatment in metastatic pancreatic adenocarcinoma
- PMID: 37201091
- PMCID: PMC10186541
- DOI: 10.21037/jgo-22-946
Performance of a blood-based RNA signature for gemcitabine-based treatment in metastatic pancreatic adenocarcinoma
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal cancer, and chemotherapy is a key treatment for advanced PDAC. Gemcitabine chemotherapy is still an important component of treatment; however, there is no routine biomarker to predict its efficacy. Predictive tests may help clinicians to decide on the best first-line chemotherapy.
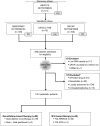
Methods: This study is a confirmatory study of a blood-based RNA signature, called the GemciTest. This test measures the expression levels of nine genes using real-time polymerase chain reaction (PCR) processes. Clinical validation was carried out, through a discovery and a validation phases, on 336 patients (mean 68.7 years; range, 37-88 years) for whom blood was collected from two prospective cohorts and two tumor biobanks. These cohorts included previously untreated advanced PDAC patients who received either a gemcitabine- or fluoropyrimidine-based regimen.
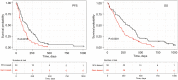
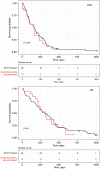
Results: Gemcitabine-based treated patients with a positive GemciTest (22.9%) had a significantly longer progression-free survival (PFS) {5.3 vs. 2.8 months; hazard ratio (HR) =0.53 [95% confidence interval (CI): 0.31-0.92]; P=0.023} and overall survival (OS) [10.4 vs. 4.8 months; HR =0.49 (95% CI: 0.29-0.85); P=0.0091]. On the contrary, fluoropyrimidine-based treated patients showed no significant difference in PFS and OS using this blood signature.
Conclusions: The GemciTest demonstrated that a blood-based RNA signature has the potential to aid in personalized therapy for PDAC, leading to better survival rates for patients receiving a gemcitabine-based first-line treatment.
Keywords: Pancreatic cancer; gemcitabine-based; liquid biopsy; predictive diagnosis.
2023 Journal of Gastrointestinal Oncology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-946/coif). DP, RB, FN, FP and A Gamez report that they are employed by Acobiom company. JBB reports that he received personal fees from Acobiom, Amgen, AstraZeneca, Bayer, Merck Serono, Pierre Fabre, Roche, Sanofi, Servier, Viatris, and non-financial support from Amgen, Merck Serono, and Roche. NKL reports that she is advisory board member for Abbvie and PDGX). The other authors have no conflicts of to declare.
Figures

Comment in
-
Determinants of gemcitabine response in pancreatic cancer: are we there?J Gastrointest Oncol. 2023 Oct 31;14(5):2290-2292. doi: 10.21037/jgo-2023-04. Epub 2023 Sep 22. J Gastrointest Oncol. 2023. PMID: 37969825 Free PMC article. No abstract available.
References
-
- Ahmad SA, Duong M, Sohal DPS, et al. Surgical Outcome Results From SWOG S1505: A Randomized Clinical Trial of mFOLFIRINOX Versus Gemcitabine/Nab-paclitaxel for Perioperative Treatment of Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg 2020;272:481-6. 10.1097/SLA.0000000000004155 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
