Inhibitory effects of vaginal Lactobacilli on C andida albicans growth, hyphal formation, biofilm development, and epithelial cell adhesion
- PMID: 37201113
- PMCID: PMC10188118
- DOI: 10.3389/fcimb.2023.1113401
Inhibitory effects of vaginal Lactobacilli on C andida albicans growth, hyphal formation, biofilm development, and epithelial cell adhesion
Abstract
Introduction: Antifungal agents are not always efficient in resolving vulvovaginal candidiasis (VVC), a common genital infection caused by the overgrowth of Candida spp., including Candida albicans, or in preventing recurrent infections. Although lactobacilli (which are dominant microorganisms constituting healthy human vaginal microbiota) are important barriers against VVC, the Lactobacillus metabolite concentration needed to suppress VVC is unknown.
Methods: We quantitatively evaluated Lactobacillus metabolite concentrations to determine their effect on Candida spp., including 27 vaginal strains of Lactobacillus crispatus, L. jensenii, L. gasseri, Lacticaseibacillus rhamnosus, and Limosilactobacillus vaginalis, with inhibitory abilities against biofilms of C. albicans clinical isolates.
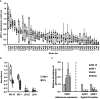
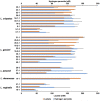
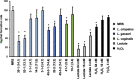
Results: Lactobacillus culture supernatants suppressed viable fungi by approximately 24%-92% relative to preformed C. albicans biofilms; however, their suppression differed among strains and not species. A moderate negative correlation was found between Lactobacillus lactate production and biofilm formation, but no correlation was observed between hydrogen peroxide production and biofilm formation. Both lactate and hydrogen peroxide were required to suppress C. albicans planktonic cell growth. Lactobacillus strains that significantly inhibited biofilm formation in culture supernatant also inhibited C. albicans adhesion to epithelial cells in an actual live bacterial adhesion competition test.
Discussion: Healthy human microflora and their metabolites may play important roles in the development of new antifungal agent against C. albicans-induced VVC.
Keywords: Candida albicans; Lactobacillus species; biofilm; cell adhesion; probiotics.
Copyright © 2023 Takano, Kudo, Eguchi, Matsumoto, Oka, Yamasaki, Takahashi, Koshikawa, Takemura, Yamagishi, Mikamo and Kunishima.
Conflict of interest statement
HaK, SE, AM, KO, and MT are employees of Miyarisan Pharmaceutical Co., Ltd.; however, they have no conflicts of interest to declare regarding this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

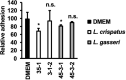
References
-
- Alves R., Mota S., Silva S., F Rodrigues C., P Brown A. J., Henriques M., et al. . (2017). The carboxylic acid transporters Jen1 and Jen2 affect the architecture and fluconazole susceptibility of Candida albicans biofilm in the presence of lactate. Biofouling 33, 943–954. doi: 10.1080/08927014.2017.1392514 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

