The value of biofilm testing to guide antimicrobial stewardship in chronic respiratory diseases
- PMID: 37201119
- PMCID: PMC10187140
- DOI: 10.3389/fcimb.2023.1142274
The value of biofilm testing to guide antimicrobial stewardship in chronic respiratory diseases
Abstract
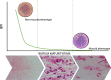
Introduction: Biofilm production is an important yet currently overlooked aspect of diagnostic microbiology that has implications for antimicrobial stewardship. In this study, we aimed to validate and identify additional applications of the BioFilm Ring Test® (BRT) for Pseudomonas aeruginosa (PA) isolates from patients with bronchiectasis (BE).
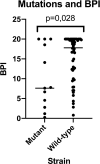
Materials and methods: Sputa were collected from BE patients who had at least one PA positive culture in the previous year. We processed the sputa to isolate both mucoid and non-mucoid PA, and determined their susceptibility pattern, mucA gene status, and presence of ciprofloxacin mutations in QRDR genes. The Biofilm production index (BPI) was obtained at 5 and 24 hours. Biofilms were imaged using Gram staining.
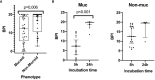
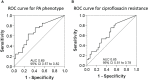
Results: We collected 69 PA isolates, including 33 mucoid and 36 non-mucoid. A BPI value below 14.75 at 5 hours predicted the mucoid PA phenotype with 64% sensitivity and 72% specificity.
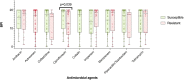
Conclusion: Overall, our findings suggest that the fitness-cost associated with the mucoid phenotype or ciprofloxacin resistance is shown through a time-dependent BPI profile. The BRT has the potential to reveal biofilm features with clinical implications.
Keywords: Pseudomonas aeruginosa; antimicrobial agents; antimicrobial resistances; biofilm; biofilm diagnose.
Copyright © 2023 Fernández-Barat, Vázquez Burgos, Alcaraz, Bueno-Freire, López-Aladid, Cabrera, Gabarrús, Palomeque, Oscanoa, Ceccato, Motos, Amaro, Bernardi, Provot, Soler-Comas, Muñoz, Vila and Torres.
Conflict of interest statement
AT has received grants from MedImmune, Cubist, Bayer, Theravance, and Polyphor and personal fees as Advisory Board member from Bayer, Roche, The Medicines CO, and Curetis. He has received bureau fees for keynote speaker presentations from GSK, Pfizer, Astra Zeneca, and Biotest Advisory Board, and are unconnected to the study submitted here. TB and CP were employed by BioFilm Pharma SAS and BioFilm Control SAS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alcaraz-Serrano V., Fernández-Barat L., Scioscia G., Llorens-Llacuna J., Gimeno-Santos E., Herrero-Cortina B., et al. . (2019). Mucoid pseudomonas aeruginosa alters sputum viscoelasticity in patients with non-cystic fibrosis bronchiectasis. Respir. Med. 154, 40–46. doi: 10.1016/j.rmed.2019.06.012 - DOI - PubMed
-
- Cabrera R., Fernández-Barat L., Vázquez N., Alcaraz-Serrano V., Bueno-Freire L., Amaro R., et al. . (2022). Resistance mechanisms and molecular epidemiology of pseudomonas aeruginosa strains from patients with bronchiectasis. J. antimicrobial chemotherapy 77 (6), 1600–1610. doi: 10.1093/jac/dkac084 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

