The cyclic lipopeptide micafungin induces rupture of isolated mitochondria by reprograming the mitochondrial inner membrane anion channel
- PMID: 37201620
- PMCID: PMC10524837
- DOI: 10.1016/j.mito.2023.05.004
The cyclic lipopeptide micafungin induces rupture of isolated mitochondria by reprograming the mitochondrial inner membrane anion channel
Abstract
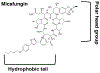
The antifungal activity of the drug micafungin, a cyclic lipopeptide that interacts with membrane proteins, may involve inhibition of fungal mitochondria. In humans, mitochondria are spared by the inability of micafungin to cross the cytoplasmic membrane. Using isolated mitochondria, we find that micafungin initiates the uptake of salts, causing rapid swelling and rupture of mitochondria with release of cytochrome c. The inner membrane anion channel (IMAC) is altered by micafungin to transfer both cations and anions. We propose that binding of anionic micafungin to IMAC attracts cations into the ion pore for the rapid transfer of ion pairs.
Keywords: Cyclic lipopeptide; Inner membrane anion channel; Ion channel; Micafungin; Mitochondrial ion channel; Mitochondrial respiratory chain complex; cytochrome c release.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
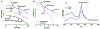
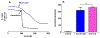
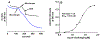
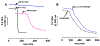
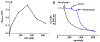

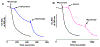
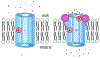
Similar articles
-
Anion uniport in plant mitochondria is mediated by a Mg(2+)-insensitive inner membrane anion channel.J Biol Chem. 1992 Feb 15;267(5):3079-87. J Biol Chem. 1992. PMID: 1371111
-
Plant inner membrane anion channel (PIMAC) function in plant mitochondria.Plant Cell Physiol. 2008 Jul;49(7):1039-55. doi: 10.1093/pcp/pcn082. Epub 2008 May 29. Plant Cell Physiol. 2008. PMID: 18511459
-
Membrane-Anchored Cyclic Peptides as Effectors of Mitochondrial Oxidative Phosphorylation.Biochemistry. 2016 Apr 12;55(14):2100-11. doi: 10.1021/acs.biochem.5b01368. Epub 2016 Mar 29. Biochemistry. 2016. PMID: 26985698
-
Properties of the inner membrane anion channel in intact mitochondria.J Bioenerg Biomembr. 1992 Feb;24(1):77-90. doi: 10.1007/BF00769534. J Bioenerg Biomembr. 1992. PMID: 1380509 Review.
-
Evidence for the existence of an inner membrane anion channel in mitochondria.Biochim Biophys Acta. 1986;853(3-4):187-204. doi: 10.1016/0304-4173(87)90001-2. Biochim Biophys Acta. 1986. PMID: 2441746 Review.
References
-
- Akar FG, Aon MA, Tomaselli GF, O’Rourke B, 2005. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115, 3527–3535, https://www.ncbi.nlm.nih.gov/pubmed/16284648. - PMC - PubMed
-
- Aon MA, Cortassa S, Maack C, O’Rourke B, 2007. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282, 21889–21900, https://www.ncbi.nlm.nih.gov/pubmed/17540766. - PMC - PubMed
-
- Aon MA, Cortassa S, Marban E, O’Rourke B, 2003. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278, 44735–44744, https://www.ncbi.nlm.nih.gov/pubmed/12930841. - PubMed
-
- Balleza D, Alessandrini A, Beltran Garcia MJ, 2019. Role of Lipid Composition, Physicochemical Interactions, and Membrane Mechanics in the Molecular Actions of Microbial Cyclic Lipopeptides. J Membr Biol 252, 131–157, https://www.ncbi.nlm.nih.gov/pubmed/31098678. - PubMed
-
- Beavis AD, 1992. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr 24, 77–90, https://www.ncbi.nlm.nih.gov/pubmed/1380509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

