PQBP1 regulates the cellular inflammation induced by avian reovirus and interacts with the viral p17 protein
- PMID: 37201645
- PMCID: PMC10345743
- DOI: 10.1016/j.virusres.2023.199119
PQBP1 regulates the cellular inflammation induced by avian reovirus and interacts with the viral p17 protein
Abstract
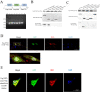
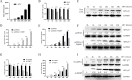
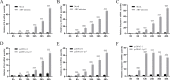
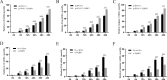
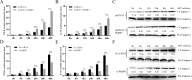
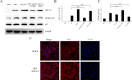
Avian reovirus (ARV) can commonly infect a flock and cause immunosuppressive diseases in poultry. The nonstructural protein p17 is involved in viral replication, and significant progress has been made in showing its ability to regulate cellular signaling pathways. In our previous study, to further investigate the effect of ARV p17 protein on viral replication, the host protein polyglu-tamine binding protein 1 (PQBP1) was identified to interact with p17 by a yeast two-hybrid system. In the current study, the interaction between PQBP1 and p17 protein was further confirmed by laser confocal microscopy and coimmunoprecipitation assays. In addition, the N-terminal WWD of PQBP1 was found to mediate the process of binding to the p17 protein. Interestingly, we found that ARV infection significantly inhibited PQBP1 expression. While the quantity of ARV replication was largely influenced by PQBP1, PQBP1 overexpression decreased ARV replication. In contrast, upon PQBP1 knockdown, the quantity of ARV was notably increased. ARV infection and p17 protein expression were both proven to induce PQBP1 to mediate cellular inflammation. In the current study, we revealed through qRT‒PCR, ELISA and Western blotting methods that PQBP1 plays a positive role in ARV-induced inflammation. Furthermore, the mechanism of this process was shown to involve the NFκB-dependent transcription of inflammatory genes. In addition, PQBP1 was shown to regulate the phosphorylation of p65 protein. In conclusion, this research provides clues to elucidating the function of the p17 protein and the pathogenic mechanism of ARV, especially the cause of the inflammatory response. It also provides new ideas for the study of therapeutic targets of ARV.
Keywords: Avian reovirus; Inflammation; P17 protein; PQBP1.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no conflict of interest!
Figures

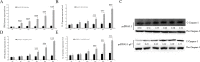
Similar articles
-
Role of PQBP1 in Pathogen Recognition-Impact on Innate Immunity.Viruses. 2024 Aug 21;16(8):1340. doi: 10.3390/v16081340. Viruses. 2024. PMID: 39205314 Free PMC article. Review.
-
IFI16 plays a critical role in avian reovirus induced cellular immunosuppression and suppresses virus replication.Poult Sci. 2024 Apr;103(4):103506. doi: 10.1016/j.psj.2024.103506. Epub 2024 Jan 30. Poult Sci. 2024. PMID: 38335672 Free PMC article.
-
Host protein PRPS2 interact with the non-structural protein p17 of Avian Reovirus and promote viral replication.Poult Sci. 2025 Jan;104(1):104582. doi: 10.1016/j.psj.2024.104582. Epub 2024 Nov 28. Poult Sci. 2025. PMID: 39631276 Free PMC article.
-
Identification and Functional Analyses of Host Proteins Interacting with the p17 Protein of Avian Reovirus.Viruses. 2022 Apr 25;14(5):892. doi: 10.3390/v14050892. Viruses. 2022. PMID: 35632635 Free PMC article.
-
Avian Reovirus in Israel, Variants and Vaccines-A Review.Avian Dis. 2022 Dec;66(4):447-451. doi: 10.1637/aviandiseases-D-22-99996. Avian Dis. 2022. PMID: 36715478 Review.
Cited by
-
Identification of Na+/K+-ATPase Inhibitor Bufalin as a Novel Pseudorabies Virus Infection Inhibitor In Vitro and In Vivo.Int J Mol Sci. 2023 Sep 23;24(19):14479. doi: 10.3390/ijms241914479. Int J Mol Sci. 2023. PMID: 37833925 Free PMC article.
-
Role of PQBP1 in Pathogen Recognition-Impact on Innate Immunity.Viruses. 2024 Aug 21;16(8):1340. doi: 10.3390/v16081340. Viruses. 2024. PMID: 39205314 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

