Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics
- PMID: 37201957
- PMCID: PMC10315697
- DOI: 10.2967/jnumed.122.265355
Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics
Abstract
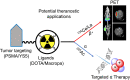
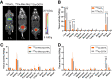
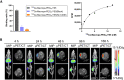
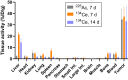
225Ac-targeted α-radiotherapy is a promising approach to treating malignancies, including prostate cancer. However, α-emitting isotopes are difficult to image because of low administered activities and a low fraction of suitable γ-emissions. The in vivo generator 134Ce/134La has been proposed as a potential PET imaging surrogate for the therapeutic nuclides 225Ac and 227Th. In this report, we detail efficient radiolabeling methods using the 225Ac-chelators DOTA and MACROPA. These methods were applied to radiolabeling of prostate cancer imaging agents, including PSMA-617 and MACROPA-PEG4-YS5, for evaluation of their in vivo pharmacokinetic characteristics and comparison to the corresponding 225Ac analogs. Methods: Radiolabeling was performed by mixing DOTA/MACROPA chelates with 134Ce/134La in NH4OAc, pH 8.0, at room temperature, and radiochemical yields were monitored by radio-thin-layer chromatography. In vivo biodistributions of 134Ce-DOTA/MACROPA.NH2 complexes were assayed through dynamic small-animal PET/CT imaging and ex vivo biodistribution studies over 1 h in healthy C57BL/6 mice, compared with free 134CeCl3 In vivo, preclinical imaging of 134Ce-PSMA-617 and 134Ce-MACROPA-PEG4-YS5 was performed on 22Rv1 tumor-bearing male nu/nu-mice. Ex vivo biodistribution was performed for 134Ce/225Ac-MACROPA-PEG4-YS5 conjugates. Results: 134Ce-MACROPA.NH2 demonstrated near-quantitative labeling with 1:1 ligand-to-metal ratios at room temperature, whereas a 10:1 ligand-to-metal ratio and elevated temperatures were required for DOTA. Rapid urinary excretion and low liver and bone uptake were seen for 134Ce/225Ac-DOTA/MACROPA. NH2 conjugates in comparison to free 134CeCl3 confirmed high in vivo stability. An interesting observation during the radiolabeling of tumor-targeting vectors PSMA-617 and MACROPA-PEG4-YS5-that the daughter 134La was expelled from the chelate after the decay of parent 134Ce-was confirmed through radio-thin-layer chromatography and reverse-phase high-performance liquid chromatography. Both conjugates, 134Ce-PSMA-617 and 134Ce-MACROPA-PEG4-YS5, displayed tumor uptake in 22Rv1 tumor-bearing mice. The ex vivo biodistribution of 134Ce-MACROPA.NH2, 134Ce-DOTA and 134Ce-MACROPA-PEG4-YS5 corroborated well with the respective 225Ac-conjugates. Conclusion: These results demonstrate the PET imaging potential for 134Ce/134La-labeled small-molecule and antibody agents. The similar 225Ac and 134Ce/134La-chemical and pharmacokinetic characteristics suggest that the 134Ce/134La pair may act as a PET imaging surrogate for 225Ac-based radioligand therapies.
Keywords: 134Ce; 225Ac; PET imaging; PSMA-617; YS5 antibody; targeted α-radiotherapy.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures



References
-
- Herrero Álvarez N, Bauer D, Hernández-Gil J, Lewis JS. Recent advances in radiometals for combined imaging and therapy in cancer. ChemMedChem. 2021;16:2909–2941. - PubMed
-
- Parker C, Lewington V, Shore N, et al. . Targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncol. 2018;4:1765–1772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
