Incidence of interstitial lung abnormalities: the MESA Lung Study
- PMID: 37202153
- PMCID: PMC10773573
- DOI: 10.1183/13993003.01950-2022
Incidence of interstitial lung abnormalities: the MESA Lung Study
Abstract
Background: The incidence of newly developed interstitial lung abnormalities (ILA) and fibrotic ILA has not been previously reported.
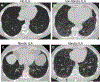
Methods: Trained thoracic radiologists evaluated 13 944 cardiac computed tomography scans for the presence of ILA in 6197 Multi-Ethnic Study of Atherosclerosis (MESA) longitudinal cohort study participants >45 years of age from 2000 to 2012. Five percent of the scans were re-read by the same or a different observer in a blinded fashion. After exclusion of participants with ILA at baseline, incidence rates and incidence rate ratios for ILA and fibrotic ILA were calculated.
Results: The intra-reader agreement of ILA was 92.0% (Gwet's AC1 0.912, intraclass correlation coefficient (ICC) 0.982) and the inter-reader agreement of ILA was 83.5% (Gwet's AC1 0.814, ICC 0.969). Incidence of ILA and fibrotic ILA was estimated to be 13.1 and 3.5 cases per 1000 person-years, respectively. In multivariable analyses, age (hazard ratio (HR) 1.06 (95% CI 1.05-1.08); p<0.001 and HR 1.08 (95% CI 1.06-1.11); p<0.001), high attenuation area at baseline (HR 1.05 (95% CI 1.03-1.07); p<0.001 and HR 1.06 (95% CI 1.02-1.10); p=0.002) and the MUC5B promoter single nucleotide polymorphism (HR 1.73 (95% CI 1.17-2.56); p=0.01 and HR 4.96 (95% CI 2.68-9.15); p<0.001) were associated with incident ILA and fibrotic ILA, respectively. Ever-smoking (HR 2.31 (95% CI 1.34-3.96); p=0.002) and an idiopathic pulmonary fibrosis polygenic risk score (HR 2.09 (95% CI 1.61-2.71); p<0.001) were associated only with incident fibrotic ILA.
Conclusions: Incident ILA and fibrotic ILA were estimated by review of cardiac imaging studies. These findings may lead to wider application of a screening tool for atherosclerosis to identify pre-clinical lung disease.
Copyright ©The authors 2023. For reproduction rights and permissions contact permissions@ersnet.org.
Conflict of interest statement
Conflict of interest: All authors report support for the present work from the National Institutes of Health. C.F. McGroder, S. Hansen, K. Hinckley Stukovsky, P.H. Nath, S.K. Sonavone, J.T. Stowell, B.M. D'Souza, J.S. Leb, S. Dumeer, M.U. Aziz, K. Batra, J.I. Rotter, A.W. Manichaikul, S.S. Rich and R.L. McClelland report no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. D. Zhang reports support for the present manuscript from Stony Wold-Herbert Fund and consulting fees from Boehringer Ingelheim, outside of this work. M.M. Salvatore reports grants, lecture honoraria, travel support and advisory board membership for Genentech and Boehringer Ingelheim, outside of this work. N. Terry reports travel support from Alabama Academy of Radiology, and acts as Treasurer, Foundation Board Member, is ACR Councillor and also reports equity in Johnson & Johnson, Kimberly-Clark Corp., Microsoft Corp., Amgen, Bristol-Myers Squibb, Cisco Systems, Medtronic, Merck, Proctor & Gamble, Crisper Therapeutics, Nvidia, Texas Instruments, Hewlett Packard, United Health, Abbott Labs, Eli Lilly and Co., AbbVie Inc. and LyondellBasell Industries. E.A. Hoffman is founder and shareholder of VIDA Diagnostics, outside of this work. E.J. Bernstein reports grants from Boehringer Ingelheim and Pfizer, and consulting fees and travel support from Boehringer Ingelheim, outside of this work. J.S. Kim reports grants from the Pulmonary Fibrosis Foundation and participation on a data safety monitoring board for the University of Virginia, outside of this work. A.J. Podolanczuk reports grants from the American Lung Association, consulting fees from Regeneron, Roche and Imvaria, lecture honoraria from the National Association for Continuing Education and EBSCO/DynaMed, and participation on an advisory board with Boehringer Ingelheim, outside of this work. D.J. Lederer reports employment and equity in Regeneron Pharmaceuticals. R.G. Barr reports grants from the American Lung Association and the COPD Foundation, and advisory board participation from the COPD Foundation, outside of this work. C.K. Garcia reports grants from the Department of Defense, NIH, Boehringer Ingelheim and AstraZeneca, and lecture honoraria from the Three Lakes Foundation, Stanford, UPenn, UCSF and Cedar Sinai, outside of this work.
Figures
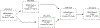

Comment in
-
How does the MESA Lung Study sharpen blurry edges in interstitial lung abnormalities?Eur Respir J. 2023 Jun 8;61(6):2300397. doi: 10.1183/13993003.00397-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37290811 No abstract available.
References
-
- Tsushima K, Sone S, Yoshikawa S, et al. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med 2010; 104: 1712–1721. - PubMed
Grants and funding
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- HHSN268201800001C/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- T32 HL105323/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- U01 HL120393/HL/NHLBI NIH HHS/United States
- R01 HL077612/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- RC1 HL100543/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- R01 HL093081/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- K23 AR075112/AR/NIAMS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- K23 HL150301/HL/NHLBI NIH HHS/United States
- R01 HL103676/HL/NHLBI NIH HHS/United States
- R01 HL117626/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
