Role of PPARγ in dyslipidemia and altered pulmonary functioning in mice following ozone exposure
- PMID: 37202362
- PMCID: PMC10306402
- DOI: 10.1093/toxsci/kfad048
Role of PPARγ in dyslipidemia and altered pulmonary functioning in mice following ozone exposure
Abstract
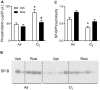
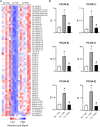
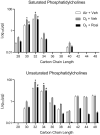
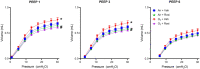
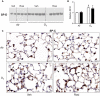
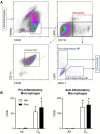
Exposure to ozone causes decrements in pulmonary function, a response associated with alterations in lung lipids. Pulmonary lipid homeostasis is dependent on the activity of peroxisome proliferator activated receptor gamma (PPARγ), a nuclear receptor that regulates lipid uptake and catabolism by alveolar macrophages (AMs). Herein, we assessed the role of PPARγ in ozone-induced dyslipidemia and aberrant lung function in mice. Exposure of mice to ozone (0.8 ppm, 3 h) resulted in a significant reduction in lung hysteresivity at 72 h post exposure; this correlated with increases in levels of total phospholipids, specifically cholesteryl esters, ceramides, phosphatidylcholines, phosphorylethanolamines, sphingomyelins, and di- and triacylglycerols in lung lining fluid. This was accompanied by a reduction in relative surfactant protein-B (SP-B) content, consistent with surfactant dysfunction. Administration of the PPARγ agonist, rosiglitazone (5 mg/kg/day, i.p.) reduced total lung lipids, increased relative amounts of SP-B, and normalized pulmonary function in ozone-exposed mice. This was associated with increases in lung macrophage expression of CD36, a scavenger receptor important in lipid uptake and a transcriptional target of PPARγ. These findings highlight the role of alveolar lipids as regulators of surfactant activity and pulmonary function following ozone exposure and suggest that targeting lipid uptake by lung macrophages may be an efficacious approach for treating altered respiratory mechanics.
Keywords: PPARγ; macrophage; ozone; pulmonary lipids.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

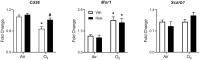
Similar articles
-
CD44 Loss Disrupts Lung Lipid Surfactant Homeostasis and Exacerbates Oxidized Lipid-Induced Lung Inflammation.Front Immunol. 2020 Jan 30;11:29. doi: 10.3389/fimmu.2020.00029. eCollection 2020. Front Immunol. 2020. PMID: 32082314 Free PMC article.
-
PPAR-gamma pathways attenuate pulmonary granuloma formation in a carbon nanotube induced murine model of sarcoidosis.Biochem Biophys Res Commun. 2018 Sep 5;503(2):684-690. doi: 10.1016/j.bbrc.2018.06.061. Epub 2018 Jun 15. Biochem Biophys Res Commun. 2018. PMID: 29908181 Free PMC article.
-
Lentivirus-ABCG1 instillation reduces lipid accumulation and improves lung compliance in GM-CSF knock-out mice.Biochem Biophys Res Commun. 2011 Nov 18;415(2):288-93. doi: 10.1016/j.bbrc.2011.10.043. Epub 2011 Oct 18. Biochem Biophys Res Commun. 2011. PMID: 22033401
-
Regulation of Lung Macrophage Activation and Oxidative Stress Following Ozone Exposure by Farnesoid X Receptor.Toxicol Sci. 2020 Oct 1;177(2):441-453. doi: 10.1093/toxsci/kfaa111. Toxicol Sci. 2020. PMID: 32984886 Free PMC article.
-
Etiology of lipid-laden macrophages in the lung.Int Immunopharmacol. 2023 Oct;123:110719. doi: 10.1016/j.intimp.2023.110719. Epub 2023 Aug 16. Int Immunopharmacol. 2023. PMID: 37595492 Free PMC article. Review.
Cited by
-
Transcriptional profiling of lung macrophages following ozone exposure in mice identifies signaling pathways regulating immunometabolic activation.Toxicol Sci. 2024 Sep 1;201(1):103-117. doi: 10.1093/toxsci/kfae081. Toxicol Sci. 2024. PMID: 38897669 Free PMC article.
-
Docosahexaenoic Acid Supplementation in Postnatal Growth Restricted Rats Does Not Normalize Lung Function or PPARγ Activity.Biomolecules. 2025 Apr 9;15(4):551. doi: 10.3390/biom15040551. Biomolecules. 2025. PMID: 40305361 Free PMC article.
-
Revealing the Molecular Mechanisms of Ozone-Induced Pulmonary Inflammatory Injury: Integrated Analysis of Metabolomics and Transcriptomics.Toxics. 2025 Apr 2;13(4):271. doi: 10.3390/toxics13040271. Toxics. 2025. PMID: 40278587 Free PMC article.
References
-
- Ahmed R. A. M., Murao K., Imachi H., Yu X., Li J., Wong N. C. W., Ishida T. (2009). Human scavenger receptor class B type 1 is regulated by activators of peroxisome proliferators-activated receptor-gamma in hepatocytes. Endocrine 35, 233–242. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

