Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria
- PMID: 37202391
- PMCID: PMC10195781
- DOI: 10.1038/s41419-023-05835-8
Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria
Abstract
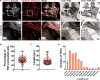
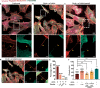
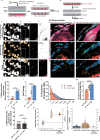
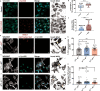
Tunnelling Nanotubes (TNTs) facilitate contact-mediated intercellular communication over long distances. Material transfer via TNTs can range from ions and intracellular organelles to protein aggregates and pathogens. Prion-like toxic protein aggregates accumulating in several neurodegenerative pathologies, such as Alzheimer's, Parkinson's, and Huntington's diseases, have been shown to spread via TNTs not only between neurons, but also between neurons-astrocytes, and neurons-pericytes, indicating the importance of TNTs in mediating neuron-glia interactions. TNT-like structures were also reported between microglia, however, their roles in neuron-microglia interaction remain elusive. In this work, we quantitatively characterise microglial TNTs and their cytoskeletal composition, and demonstrate that TNTs form between human neuronal and microglial cells. We show that α-Synuclein (α-Syn) aggregates increase the global TNT-mediated connectivity between cells, along with the number of TNT connections per cell pair. Homotypic TNTs formed between microglial cells, and heterotypic TNTs between neuronal and microglial cells are furthermore shown to be functional, allowing movement of both α-Syn and mitochondria. Quantitative analysis shows that α-Syn aggregates are transferred predominantly from neuronal to microglial cells, possibly as a mechanism to relieve the burden of accumulated aggregates. By contrast, microglia transfer mitochondria preferably to α-Syn burdened neuronal cells over the healthy ones, likely as a potential rescue mechanism. Besides describing novel TNT-mediated communication between neuronal and microglial cells, this work allows us to better understand the cellular mechanisms of spreading neurodegenerative diseases, shedding light on the role of microglia.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes.Neuron. 2024 Sep 25;112(18):3106-3125.e8. doi: 10.1016/j.neuron.2024.06.029. Epub 2024 Jul 25. Neuron. 2024. PMID: 39059388
-
Human Astrocytes Transfer Aggregated Alpha-Synuclein via Tunneling Nanotubes.J Neurosci. 2017 Dec 6;37(49):11835-11853. doi: 10.1523/JNEUROSCI.0983-17.2017. Epub 2017 Oct 31. J Neurosci. 2017. PMID: 29089438 Free PMC article.
-
α-synuclein transfer through tunneling nanotubes occurs in SH-SY5Y cells and primary brain pericytes from Parkinson's disease patients.Sci Rep. 2017 Feb 23;7:42984. doi: 10.1038/srep42984. Sci Rep. 2017. PMID: 28230073 Free PMC article.
-
A new hypothesis of pathogenesis based on the divorce between mitochondria and their host cells: possible relevance for Alzheimer's disease.Curr Alzheimer Res. 2010 Jun;7(4):307-22. doi: 10.2174/156720510791162395. Curr Alzheimer Res. 2010. PMID: 19860724 Review.
-
Hijacking intercellular trafficking for the spread of protein aggregates in neurodegenerative diseases: a focus on tunneling nanotubes (TNTs).Extracell Vesicles Circ Nucl Acids. 2023 Mar 9;4(1):27-43. doi: 10.20517/evcna.2023.05. eCollection 2023. Extracell Vesicles Circ Nucl Acids. 2023. PMID: 39698299 Free PMC article. Review.
Cited by
-
Intercellular crosstalk mediated by tunneling nanotubes between central nervous system cells. What we need to advance.Front Physiol. 2023 Aug 21;14:1214210. doi: 10.3389/fphys.2023.1214210. eCollection 2023. Front Physiol. 2023. PMID: 37670766 Free PMC article. Review.
-
α-Synuclein Degradation in Brain Pericytes Is Mediated via Akt, ERK, and p38 MAPK Signaling Pathways.Int J Mol Sci. 2025 Feb 14;26(4):1615. doi: 10.3390/ijms26041615. Int J Mol Sci. 2025. PMID: 40004079 Free PMC article.
-
Tunneling Nanotube: An Enticing Cell-Cell Communication in the Nervous System.Biology (Basel). 2023 Sep 27;12(10):1288. doi: 10.3390/biology12101288. Biology (Basel). 2023. PMID: 37886998 Free PMC article. Review.
-
Protective effect of mesenchymal stromal cells in diabetic nephropathy: the In vitro and In vivo role of the M-Sec-tunneling nanotubes.Clin Sci (Lond). 2024 Dec 4;138(23):1537-1559. doi: 10.1042/CS20242064. Clin Sci (Lond). 2024. PMID: 39535903 Free PMC article.
-
Combining sophisticated fast FLIM, confocal microscopy, and STED nanoscopy for live-cell imaging of tunneling nanotubes.Life Sci Alliance. 2024 Apr 22;7(7):e202302398. doi: 10.26508/lsa.202302398. Print 2024 Jul. Life Sci Alliance. 2024. PMID: 38649185 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

