Debiased ambient vibrations optical coherence elastography to profile cell, organoid and tissue mechanical properties
- PMID: 37202417
- PMCID: PMC10195840
- DOI: 10.1038/s42003-023-04788-0
Debiased ambient vibrations optical coherence elastography to profile cell, organoid and tissue mechanical properties
Abstract
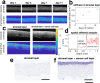
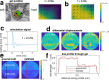
The role of the mechanical environment in defining tissue function, development and growth has been shown to be fundamental. Assessment of the changes in stiffness of tissue matrices at multiple scales has relied mostly on invasive and often specialist equipment such as AFM or mechanical testing devices poorly suited to the cell culture workflow.In this paper, we have developed a unbiased passive optical coherence elastography method, exploiting ambient vibrations in the sample that enables real-time noninvasive quantitative profiling of cells and tissues. We demonstrate a robust method that decouples optical scattering and mechanical properties by actively compensating for scattering associated noise bias and reducing variance. The efficiency for the method to retrieve ground truth is validated in silico and in vitro, and exemplified for key applications such as time course mechanical profiling of bone and cartilage spheroids, tissue engineering cancer models, tissue repair models and single cell. Our method is readily implementable with any commercial optical coherence tomography system without any hardware modifications, and thus offers a breakthrough in on-line tissue mechanical assessment of spatial mechanical properties for organoids, soft tissues and tissue engineering.
© 2023. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Guimarães CF, Gasperini L, Marques AP, Reis RL. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020;5:351–370. doi: 10.1038/s41578-019-0169-1. - DOI
-
- Wall M, et al. Key developments that impacted the field of mechanobiology and mechanotransduction. J. Orthop. Res. 2018;36:605–619. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

