Nanoparticle-based targeting of microglia improves the neural regeneration enhancing effects of immunosuppression in the zebrafish retina
- PMID: 37202450
- PMCID: PMC10193316
- DOI: 10.1038/s42003-023-04898-9
Nanoparticle-based targeting of microglia improves the neural regeneration enhancing effects of immunosuppression in the zebrafish retina
Abstract
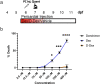
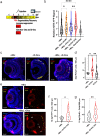
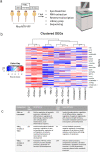
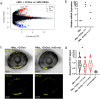
Retinal Müller glia function as injury-induced stem-like cells in zebrafish but not mammals. However, insights gleaned from zebrafish have been applied to stimulate nascent regenerative responses in the mammalian retina. For instance, microglia/macrophages regulate Müller glia stem cell activity in the chick, zebrafish, and mouse. We previously showed that post-injury immunosuppression by the glucocorticoid dexamethasone accelerated retinal regeneration kinetics in zebrafish. Similarly, microglia ablation enhances regenerative outcomes in the mouse retina. Targeted immunomodulation of microglia reactivity may therefore enhance the regenerative potential of Müller glia for therapeutic purposes. Here, we investigated potential mechanisms by which post-injury dexamethasone accelerates retinal regeneration kinetics, and the effects of dendrimer-based targeting of dexamethasone to reactive microglia. Intravital time-lapse imaging revealed that post-injury dexamethasone inhibited microglia reactivity. The dendrimer-conjugated formulation: (1) decreased dexamethasone-associated systemic toxicity, (2) targeted dexamethasone to reactive microglia, and (3) improved the regeneration enhancing effects of immunosuppression by increasing stem/progenitor proliferation rates. Lastly, we show that the gene rnf2 is required for the enhanced regeneration effect of D-Dex. These data support the use of dendrimer-based targeting of reactive immune cells to reduce toxicity and enhance the regeneration promoting effects of immunosuppressants in the retina.
© 2023. The Author(s).
Conflict of interest statement
J.S.M. holds patents for the NTR inducible cell ablation system (US #7,514,595) and uses thereof (US #8,071,838 and US#8431768). R.M.K. and S.P.K. have been awarded pending patents relating to the hydroxyl dendrimer platform for ocular therapies. RMK and his wife (Sujatha Kannan) are co-founders/board members and have financial interests in Ashvattha Therapeutics Inc., a start-up focusing on clinical translation of the dendrimer platform. The remaining authors declare no competing interests.
Figures


References
-
- Yurco, P. & Cameron, D. A. Responses of Müller glia to retinal injury in adult zebrafish. Vis. Res. 10.1016/j.visres.2004.10.022 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

