Refined high-content imaging-based phenotypic drug screening in zebrafish xenografts
- PMID: 37202469
- PMCID: PMC10195872
- DOI: 10.1038/s41698-023-00386-9
Refined high-content imaging-based phenotypic drug screening in zebrafish xenografts
Abstract
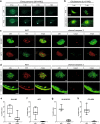
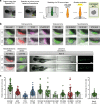
Zebrafish xenotransplantation models are increasingly applied for phenotypic drug screening to identify small compounds for precision oncology. Larval zebrafish xenografts offer the opportunity to perform drug screens at high-throughput in a complex in vivo environment. However, the full potential of the larval zebrafish xenograft model has not yet been realized and several steps of the drug screening workflow still await automation to increase throughput. Here, we present a robust workflow for drug screening in zebrafish xenografts using high-content imaging. We established embedding methods for high-content imaging of xenografts in 96-well format over consecutive days. In addition, we provide strategies for automated imaging and analysis of zebrafish xenografts including automated tumor cell detection and tumor size analysis over time. We also compared commonly used injection sites and cell labeling dyes and show specific site requirements for tumor cells from different entities. We demonstrate that our setup allows us to investigate proliferation and response to small compounds in several zebrafish xenografts ranging from pediatric sarcomas and neuroblastoma to glioblastoma and leukemia. This fast and cost-efficient assay enables the quantification of anti-tumor efficacy of small compounds in large cohorts of a vertebrate model system in vivo. Our assay may aid in prioritizing compounds or compound combinations for further preclinical and clinical investigations.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- WHO, “Cancer”, https://www.who.int/news-room/fact-sheets/detail/cancer (2022).
Publication types
Grants and funding
- 7940628 533/Österreichische Forschungsförderungsgesellschaft (Austrian Research Promotion Agency)
- SI 29/2023/OeAD-GmbH (OEAD)
- DOC fellowship/Österreichischen Akademie der Wissenschaften (Austrian Academy of Sciences)
- LS18-111/Vienna Science and Technology Fund (Wiener Wissenschafts-, Forschungs- und Technologiefonds)
- 116064-ITCC P4/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Research Materials

