Guidelines for genetic testing in prostate cancer: a scoping review
- PMID: 37202470
- PMCID: PMC11543603
- DOI: 10.1038/s41391-023-00676-0
Guidelines for genetic testing in prostate cancer: a scoping review
Abstract
Background: Genetic testing, to identify pathogenic or likely pathogenic variants in prostate cancer, is valuable in guiding treatment decisions for men with prostate cancer and to inform cancer prevention and early detection options for their immediate blood relatives. There are various guidelines and consensus statements for genetic testing in prostate cancer. Our aim is to review genetic testing recommendations across current guidelines and consensus statements and the level of evidence supporting those recommendations.
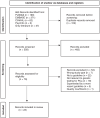
Methods: A scoping review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping review (PRISMA-ScR) guidelines. Electronic database searches and manual searches of grey literature, including websites of key organisations were conducted. Using the Population, Concept, Context (PCC) framework, this scoping review included: men with prostate cancer or men at high risk of prostate cancer and their biological families; existing guidelines and consensus statements with supporting evidence for genetic testing of men with prostate cancer from any geographical location worldwide.
Results: Of the 660 citations identified, 23 guidelines and consensus statements met the inclusion criteria for the scoping review. Based on different levels of evidence about who should be tested and how, a diverse range of recommendations were identified. There was general consensus among the guidelines and consensus statements that men with metastatic disease be offered genetic testing; however, there was less consensus in relation to genetic testing in localised prostate cancer. While there was some consensus in relation to which genes to test, recommendations varied regarding who to test, testing methods and implementation.
Conclusion: While genetic testing in prostate cancer is routinely recommended and numerous guidelines exist, there is still considerable lack of consensus regarding who should be tested and how they should be tested. Further evidence is needed to inform value-based genetic testing strategies for implementation in practice.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Cancer Screening and Genetic Testing Recommendations for Relatives of Men Undergoing Prostate Cancer Germline Testing: Implications for Practice.J Urol. 2020 Dec;204(6):1116-1118. doi: 10.1097/JU.0000000000001150. Epub 2020 May 27. J Urol. 2020. PMID: 32926644 No abstract available.
-
Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017.J Clin Oncol. 2018 Feb 1;36(4):414-424. doi: 10.1200/JCO.2017.74.1173. Epub 2017 Dec 13. J Clin Oncol. 2018. PMID: 29236593 Free PMC article. Review.
-
Clinical Implications of Germline Testing in Newly Diagnosed Prostate Cancer.Eur Urol Oncol. 2021 Feb;4(1):1-9. doi: 10.1016/j.euo.2020.11.011. Epub 2020 Dec 31. Eur Urol Oncol. 2021. PMID: 33390340 Review.
-
Systematic Review of Active Surveillance for Clinically Localised Prostate Cancer to Develop Recommendations Regarding Inclusion of Intermediate-risk Disease, Biopsy Characteristics at Inclusion and Monitoring, and Surveillance Repeat Biopsy Strategy.Eur Urol. 2022 Apr;81(4):337-346. doi: 10.1016/j.eururo.2021.12.007. Epub 2021 Dec 31. Eur Urol. 2022. PMID: 34980492
Cited by
-
Deciphering Complexity: The Molecular Landscape of Castration-Resistant Prostate Cancer.Surg Pathol Clin. 2025 Mar;18(1):25-39. doi: 10.1016/j.path.2024.10.003. Epub 2024 Nov 29. Surg Pathol Clin. 2025. PMID: 39890307 Review.
-
Using a behaviour-change approach to support uptake of population genomic screening and management options for breast or prostate cancer.Eur J Hum Genet. 2025 Jan;33(1):108-120. doi: 10.1038/s41431-024-01729-1. Epub 2024 Nov 12. Eur J Hum Genet. 2025. PMID: 39532988
-
Predictive Value and Therapeutic Significance of Somatic BRCA Mutation in Solid Tumors.Biomedicines. 2024 Mar 6;12(3):593. doi: 10.3390/biomedicines12030593. Biomedicines. 2024. PMID: 38540206 Free PMC article. Review.
-
Combination of PARP Inhibitors and Androgen Receptor Pathway Inhibitors in Metastatic Castration-Resistant Prostate Cancer.Drugs. 2024 Sep;84(9):1093-1109. doi: 10.1007/s40265-024-02071-y. Epub 2024 Jul 26. Drugs. 2024. PMID: 39060912 Free PMC article. Review.
-
BRCA Mutation Testing in Men With Metastatic Castration-Resistant Prostate Cancer: Practical Guidance for Australian Clinical Practice.Asia Pac J Clin Oncol. 2025 Aug;21(4):345-358. doi: 10.1111/ajco.14150. Epub 2025 Jan 18. Asia Pac J Clin Oncol. 2025. PMID: 39825869 Free PMC article. Review.
References
-
- Sung HFJ, Siegel RL, Laversanne M, Soerjomatara I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clinicians. 2021;71:209–49. - PubMed
-
- Roehrborn C, Black L. The economic burden of prostate cancer. BJUI Int. 2011;108:806–13. - PubMed
-
- Australian Institute of Health and Welfare. Cancer data in Australia. Canberra: AIHW; 2022.
-
- Australian Institute of Health and Welfare. Health system expenditure on cancer and other neoplasms in Australia, 2015–16. Canberra: AIHW; 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous