Role of ferroptosis in the pathogenesis and as a therapeutic target of inflammatory bowel disease (Review)
- PMID: 37203397
- PMCID: PMC10198063
- DOI: 10.3892/ijmm.2023.5256
Role of ferroptosis in the pathogenesis and as a therapeutic target of inflammatory bowel disease (Review)
Abstract
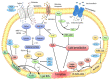
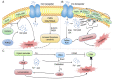
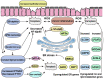
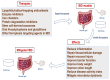
Ferroptosis, a novel form of regulated cell death, is characterized by the accumulation of labile iron and lipid peroxidation, and the excessive production of reactive oxygen species (ROS). Although ferroptosis lies at the center of crucial biological activities involving O2, iron and polyunsaturated fatty acids (PUFAs), which are essential for cell proliferation and growth, the interaction between these molecules could also mediate the accumulation of toxic levels of ROS and lipid peroxides, which can then cause damage to cellular membranes and ultimately result in cell death. Recent reports have indicated that ferroptosis participates in the development and progression of inflammatory bowel disease (IBD), offering a new exploratory field which may aid in the more in‑depth understanding of the pathogenesis and therapeutic targets of IBD. Of note, the mitigation of the characteristic features of ferroptosis, such as depleted glutathione (GSH) levels, inactivated glutathione peroxidase 4 (GPX4), elevated levels of lipid peroxidation and iron overload significantly relieve IBD. This has attracted the attention of researches aiming to examine therapeutic agents that inhibit ferroptosis in IBD, including radical‑trapping antioxidants, enzyme inhibitors, iron chelators, protein degradation inhibitors, stem cell‑derived exosomes and oral N‑acetylcysteine or glutathione. The present review summarizes and discusses the current data that implicate ferroptosis in the pathogenesis of IBD and its inhibition as a novel alternate therapeutic target for IBD. The mechanisms and key mediators of ferroptosis, including GSH/GPX4, PUFAs, iron and organic peroxides are also discussed. Although the field is relatively new, the therapeutic regulation of ferroptosis has exhibited promising outcomes as a novel treatment avenue for IBD.
Keywords: ferroptosis; glutathione peroxidase 4; inflammatory bowel disease; intestinal disease; iron overload; lipid peroxidation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ocansey DKW, Xu X, Zhang L, Mao F. Mesenchymal stem cell-derived exosome: The likely game-changer in stem cell research. BIOCELL. 2022;46:1169–1172. doi: 10.32604/biocell.2022.018470. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

