Making memories last using the peripheral effect of direct current stimulation
- PMID: 37204308
- PMCID: PMC10241520
- DOI: 10.7554/eLife.75586
Making memories last using the peripheral effect of direct current stimulation
Abstract
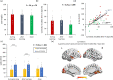
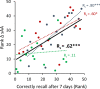
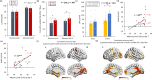
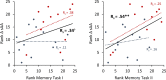
Most memories that are formed are forgotten, while others are retained longer and are subject to memory stabilization. We show that non-invasive transcutaneous electrical stimulation of the greater occipital nerve (NITESGON) using direct current during learning elicited a long-term memory effect. However, it did not trigger an immediate effect on learning. A neurobiological model of long-term memory proposes a mechanism by which memories that are initially unstable can be strengthened through subsequent novel experiences. In a series of studies, we demonstrate NITESGON's capability to boost the retention of memories when applied shortly before, during, or shortly after the time of learning by enhancing memory consolidation via activation and communication in and between the locus coeruleus pathway and hippocampus by plausibly modulating dopaminergic input. These findings may have a significant impact for neurocognitive disorders that inhibit memory consolidation such as Alzheimer's disease.
Keywords: behavioral tagging; brain stimulation; dopamine; human; interference; locus coeruleus; neuroscience.
© 2023, Luckey et al.
Conflict of interest statement
AL, LM, YH, AM, SV No competing interests declared
Figures








Comment in
-
Let's Shape Learning Into Lasting Memories.Neurosci Insights. 2024 Feb 9;19:26331055241227220. doi: 10.1177/26331055241227220. eCollection 2024. Neurosci Insights. 2024. PMID: 38343791 Free PMC article.
References
-
- Astafiev SV, Snyder AZ, Shulman GL, Corbetta M. Comment on "Modafinil shifts human locus coeruleus to low-tonic, high-Phasic activity during functional MRI" and "Homeostatic sleep pressure and responses to sustained attention in the Suprachiasmatic area. Science. 2010;328:309. doi: 10.1126/science.1177948. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

