Effect of Contact Lens Solutions in Stabilizing the Activity of Tear Lysozyme
- PMID: 37205004
- PMCID: PMC10187650
- DOI: 10.2147/OPTO.S404261
Effect of Contact Lens Solutions in Stabilizing the Activity of Tear Lysozyme
Abstract
Purpose: Interactions between tear proteins and the interfaces of contact lenses can be complex and can influence contact lens wear success. Tear proteins, including lysozyme, function to maintain the balance of ocular surface homeostasis, as evidenced by the effects of its conformation relative to stabilizing the tear film and its potential impact on corneal epithelial cells. Contact lens manufacturers include components in lens care and blister package solutions to help stabilize the tear film and preserve homeostasis. This in vitro study was performed to evaluate the ability of daily disposable contact lens package solutions to stabilize lysozyme and preserve its native conformation under denaturing conditions.
Methods: Lysozyme was added to contact lens solutions sampled from kalifilcon A, etafilcon A, senofilcon A, narafilcon A, nelfilcon A, verofilcon A, delefilcon A, somofilcon A, and stenfilcon A blister packages, then mixed with the protein denaturant sodium lauryl sulfate. Lysozyme activity was evaluated by adding test solutions to a suspension of Micrococcus luteus. Native lysozyme lyses the Micrococcus luteus cell wall, which decreases suspension turbidity. Stabilization of lysozyme activity was determined by comparing suspension turbidity before and after exposure to test solutions.
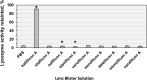
Results: Lysozyme stabilization was 90.7% for kalifilcon A solution, a statistically significant improvement (p < 0.05) compared to phosphate buffered saline (PBS, negative control). No significant improvement was observed with any other contact lens solution (all lysozyme stabilization < 5.00%).
Conclusion: The representative tear protein lysozyme was significantly more stable in the novel kalifilcon A contact lens solution containing multiple moisturizers and osmoprotectants than in PBS or other daily disposable contact lens solutions. The lysozyme activity assay provides mechanistic evidence that the kalifilcon A contact lens solution can stabilize proteins under conditions that typically denature proteins, which may contribute to maintaining ocular surface homeostasis.
Keywords: homeostasis; kalifilcon A; protein stabilization; silicone hydrogel.
© 2023 Scheuer et al.
Conflict of interest statement
All authors are employees of Bausch & Lomb Incorporated. Ms Catherine A Scheuer reports a patent 17/398556 pending to Bausch+Lomb, a patent 110129328 pending to Bausch+Lomb, a patent PCT/EP2021/072140 pending to Bausch+Lomb. Ms Vicki L Barniak reports a patent 17/398,556 pending to Bausch & Lomb, a patent 110129328 pending to Bausch & Lomb, a patent PCT/EP2021/072140 pending to Bausch & Lomb. Dr William Reindel reports a patent 17/398556 pending to Bausch+Lomb, a patent 110129328 pending to Bausch+Lomb, a patent PCT/EP2021/072140 pending to Bausch+Lomb. The authors report no other conflicts of interest in this work.
Figures

References
-
- biologyonline.com. Homeostasis; 2022. Available from: https://www.biologyonline.com/dictionary/homeostasis. Accessed April 4, 2023.
LinkOut - more resources
Full Text Sources

