Seroprevalence and durability of antibody responses to AstraZeneca vaccination in Ugandans with prior mild or asymptomatic COVID-19: implications for vaccine policy
- PMID: 37205095
- PMCID: PMC10187141
- DOI: 10.3389/fimmu.2023.1183983
Seroprevalence and durability of antibody responses to AstraZeneca vaccination in Ugandans with prior mild or asymptomatic COVID-19: implications for vaccine policy
Abstract
Introduction: The duration and timing of immunity conferred by COVID-19 vaccination in sub-Saharan Africa are crucial for guiding pandemic policy interventions, but systematic data for this region is scarce. This study investigated the antibody response after AstraZeneca vaccination in COVID-19 convalescent Ugandans.
Methods: We recruited 86 participants with a previous rt-PCR-confirmed mild or asymptomatic COVID-19 infection and measured the prevalence and levels of spike-directed IgG, IgM, and IgA antibodies at baseline, 14 and 28 days after the first dose (priming), 14 days after the second dose (boosting), and at six- and nine-months post-priming. We also measured the prevalence and levels of nucleoprotein-directed antibodies to assess breakthrough infections.
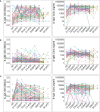
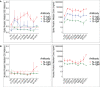
Results: Within two weeks of priming, vaccination substantially increased the prevalence and concentrations of spike-directed antibodies (p < 0.0001, Wilcoxon signed rank test), with 97.0% and 66% of vaccinated individuals possessing S-IgG and S-IgA antibodies before administering the booster dose. S-IgM prevalence changed marginally after the initial vaccination and barely after the booster, consistent with an already primed immune system. However, we also observed a rise in nucleoprotein seroprevalence, indicative of breakthroughs six months after the initial vaccination.
Discussion: Our results suggest that vaccination of COVID-19 convalescent individuals with the AstraZeneca vaccine induces a robust and differential spike-directed antibody response. The data highlights the value of vaccination as an effective method for inducing immunity in previously infected individuals and the importance of administering two doses to maintain protective immunity. Monitoring anti-spike IgG and IgA when assessing vaccine-induced antibody responses is suggested for this population; assessing S-IgM will underestimate the response. The AstraZeneca vaccine is a valuable tool in the fight against COVID-19. Further research is needed to determine the durability of vaccine-induced immunity and the potential need for booster doses.
Keywords: AstraZeneca vaccine; IgG; IgM; Ugandan population; and IgA antibody responses; hybrid immunity; nucleoprotein-directed antibodies; spike-directed antibodies.
Copyright © 2023 Serwanga, Baine, Mugaba, Ankunda, Auma, Oluka, Kato, Kitabye, Sembera, Odoch, Ejou, Nalumansi, Gombe, Musenero, Kaleebu and the COVID-19 Immunoprofiling Team.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. . Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis (2023) 22:S1473-3099(22)00801-5. doi: 10.1016/S1473-3099(22)00801-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

