The relationship between gut microbiota and COVID-19 progression: new insights into immunopathogenesis and treatment
- PMID: 37205106
- PMCID: PMC10185909
- DOI: 10.3389/fimmu.2023.1180336
The relationship between gut microbiota and COVID-19 progression: new insights into immunopathogenesis and treatment
Abstract
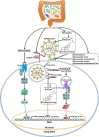
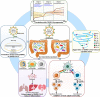
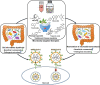
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a global health crisis. Increasing evidence underlines the key role of competent immune responses in resisting SARS-CoV-2 infection and manifests the disastrous consequence of host immune dysregulation. Elucidating the mechanisms responsible for deregulated host immunity in COVID-19 may provide a theoretical basis for further research on new treatment modalities. Gut microbiota comprises trillions of microorganisms colonizing the human gastrointestinal tract and has a vital role in immune homeostasis and the gut-lung crosstalk. Particularly, SARS-CoV-2 infection can lead to the disruption of gut microbiota equilibrium, a condition called gut dysbiosis. Due to its regulatory effect on host immunity, gut microbiota has recently received considerable attention in the field of SARS-CoV-2 immunopathology. Imbalanced gut microbiota can fuel COVID-19 progression through production of bioactive metabolites, intestinal metabolism, enhancement of the cytokine storm, exaggeration of inflammation, regulation of adaptive immunity and other aspects. In this review, we provide an overview of the alterations in gut microbiota in COVID-19 patients, and their effects on individuals' susceptibility to viral infection and COVID-19 progression. Moreover, we summarize currently available data on the critical role of the bidirectional regulation between intestinal microbes and host immunity in SARS-CoV-2-induced pathology, and highlight the immunomodulatory mechanisms of gut microbiota contributing to COVID-19 pathogenesis. In addition, we discuss the therapeutic benefits and future perspectives of microbiota-targeted interventions including faecal microbiota transplantation (FMT), bacteriotherapy and traditional Chinese medicine (TCM) in COVID-19 treatment.
Keywords: COVID-19; adaptive immunity; cytokine storm; gut microbiota; inflammation; microbiota-targeted interventions.
Copyright © 2023 Wang, Zhang, Li, Chang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
COVID-19 influenced gut dysbiosis, post-acute sequelae, immune regulation, and therapeutic regimens.Front Cell Infect Microbiol. 2024 May 28;14:1384939. doi: 10.3389/fcimb.2024.1384939. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38863829 Free PMC article. Review.
-
Traditional Chinese medicine against COVID-19: Role of the gut microbiota.Biomed Pharmacother. 2022 May;149:112787. doi: 10.1016/j.biopha.2022.112787. Epub 2022 Mar 8. Biomed Pharmacother. 2022. PMID: 35279010 Free PMC article. Review.
-
Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications.Nat Rev Gastroenterol Hepatol. 2023 May;20(5):323-337. doi: 10.1038/s41575-022-00698-4. Epub 2022 Oct 21. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36271144 Free PMC article. Review.
-
Roles of the gut microbiota in severe SARS-CoV-2 infection.Cytokine Growth Factor Rev. 2022 Feb;63:98-107. doi: 10.1016/j.cytogfr.2022.01.007. Epub 2022 Jan 31. Cytokine Growth Factor Rev. 2022. PMID: 35131164 Free PMC article. Review.
-
Alterations of the gut microbiota in coronavirus disease 2019 and its therapeutic potential.World J Gastroenterol. 2022 Dec 21;28(47):6689-6701. doi: 10.3748/wjg.v28.i47.6689. World J Gastroenterol. 2022. PMID: 36620345 Free PMC article. Review.
Cited by
-
The pediatric gut bacteriome and virome in response to SARS-CoV-2 infection.Front Cell Infect Microbiol. 2024 Jan 22;14:1335450. doi: 10.3389/fcimb.2024.1335450. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38318164 Free PMC article.
-
Global deletion of the immune cell transcription factor, T-bet, alters gut microbiota and insulin sensitivity in mice.Front Genet. 2024 Nov 27;15:1502832. doi: 10.3389/fgene.2024.1502832. eCollection 2024. Front Genet. 2024. PMID: 39664730 Free PMC article.
-
The Gut-Organ Axis within the Human Body: Gut Dysbiosis and the Role of Prebiotics.Life (Basel). 2023 Oct 8;13(10):2023. doi: 10.3390/life13102023. Life (Basel). 2023. PMID: 37895405 Free PMC article. Review.
-
COVID-19 influenced gut dysbiosis, post-acute sequelae, immune regulation, and therapeutic regimens.Front Cell Infect Microbiol. 2024 May 28;14:1384939. doi: 10.3389/fcimb.2024.1384939. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38863829 Free PMC article. Review.
-
Excess of body weight is associated with accelerated T-cell senescence in hospitalized COVID-19 patients.Immun Ageing. 2024 Mar 8;21(1):17. doi: 10.1186/s12979-024-00423-6. Immun Ageing. 2024. PMID: 38454515 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

