This is a preprint.
Histone H3 serotonylation dynamics in dorsal raphe nucleus contribute to stress- and antidepressant-mediated gene expression and behavior
- PMID: 37205414
- PMCID: PMC10187276
- DOI: 10.1101/2023.05.04.539464
Histone H3 serotonylation dynamics in dorsal raphe nucleus contribute to stress- and antidepressant-mediated gene expression and behavior
Update in
-
Histone serotonylation in dorsal raphe nucleus contributes to stress- and antidepressant-mediated gene expression and behavior.Nat Commun. 2024 Jun 13;15(1):5042. doi: 10.1038/s41467-024-49336-4. Nat Commun. 2024. PMID: 38871707 Free PMC article.
Abstract
Background: Major depressive disorder (MDD), along with related mood disorders, is a debilitating illness that affects millions of individuals worldwide. While chronic stress increases incidence levels of mood disorders, stress-mediated disruptions in brain function that precipitate these illnesses remain elusive. Serotonin-associated antidepressants (ADs) remain the first line of therapy for many with depressive symptoms, yet low remission rates and delays between treatment and symptomatic alleviation have prompted skepticism regarding precise roles for serotonin in the precipitation of mood disorders. Our group recently demonstrated that serotonin epigenetically modifies histone proteins (H3K4me3Q5ser) to regulate transcriptional permissiveness in brain. However, this phenomenon has not yet been explored following stress and/or AD exposures.
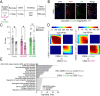
Methods: We employed a combination of genome-wide and biochemical analyses in dorsal raphe nucleus (DRN) of male and female mice exposed to chronic social defeat stress to examine the impact of stress exposures on H3K4me3Q5ser dynamics, as well as associations between the mark and stress-induced gene expression. We additionally assessed stress-induced regulation of H3K4me3Q5ser following AD exposures, and employed viral-mediated gene therapy to reduce H3K4me3Q5ser levels in DRN and examine the impact on stress-associated gene expression and behavior.
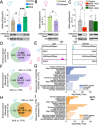
Results: We found that H3K4me3Q5ser plays important roles in stress-mediated transcriptional plasticity. Chronically stressed mice displayed dysregulated H3K4me3Q5ser dynamics in DRN, with both AD- and viral-mediated disruption of these dynamics proving sufficient to rescue stress-mediated gene expression and behavior.
Conclusions: These findings establish a neurotransmission-independent role for serotonin in stress-/AD-associated transcriptional and behavioral plasticity in DRN.
Keywords: ChIP-seq; Chronic social defeat stress; RNA-seq; antidepressants; dorsal raphe nucleus; histone serotonylation.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures


References
-
- Warnick SJ, Mehdi L, Kowalkowski J (2021): Wait-there’s evidence for that? Integrative medicine treatments for major depressive disorder. Int J Psychiatry Med 56: 334–343. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
