This is a preprint.
Keratin isoform shifts modulate motility signals during wound healing
- PMID: 37205459
- PMCID: PMC10187270
- DOI: 10.1101/2023.05.04.538989
Keratin isoform shifts modulate motility signals during wound healing
Update in
-
Shifts in keratin isoform expression activate motility signals during wound healing.Dev Cell. 2024 Oct 21;59(20):2759-2771.e11. doi: 10.1016/j.devcel.2024.06.011. Epub 2024 Jul 12. Dev Cell. 2024. PMID: 39002537 Free PMC article.
Abstract
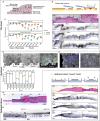
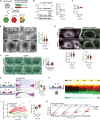
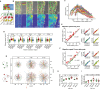
Keratin intermediate filaments form strong mechanical scaffolds that confer structural stability to epithelial tissues, but the reason this function requires a protein family with 54 isoforms is not understood. During skin wound healing, a shift in keratin isoform expression alters the composition of keratin filaments. How this change modulates cellular function to support epidermal remodeling remains unclear. We report an unexpected effect of keratin isoform variation on kinase signal transduction. Increased expression of wound-associated keratin 6A, but not of steady-state keratin 5, potentiated keratinocyte migration and wound closure without compromising epidermal stability by activating myosin motors. This pathway depended on isoform-specific interaction between intrinsically disordered keratin head domains and non-filamentous vimentin shuttling myosin-activating kinases. These results substantially expand the functional repertoire of intermediate filaments from their canonical role as mechanical scaffolds to include roles as isoform-tuned signaling scaffolds that organize signal transduction cascades in space and time to influence epithelial cell state.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
