This is a preprint.
Conserved cardiolipin-mitochondrial ADP/ATP carrier interactions assume distinct structural and functional roles that are clinically relevant
- PMID: 37205478
- PMCID: PMC10187269
- DOI: 10.1101/2023.05.05.539595
Conserved cardiolipin-mitochondrial ADP/ATP carrier interactions assume distinct structural and functional roles that are clinically relevant
Update in
-
Functional diversity among cardiolipin binding sites on the mitochondrial ADP/ATP carrier.EMBO J. 2024 Jul;43(14):2979-3008. doi: 10.1038/s44318-024-00132-2. Epub 2024 Jun 5. EMBO J. 2024. PMID: 38839991 Free PMC article.
Abstract
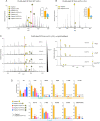
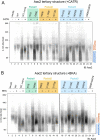
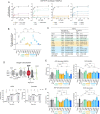
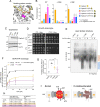
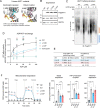
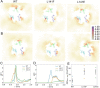
The mitochondrial phospholipid cardiolipin (CL) promotes bioenergetics via oxidative phosphorylation (OXPHOS). Three tightly bound CLs are evolutionarily conserved in the ADP/ATP carrier (AAC in yeast; adenine nucleotide translocator, ANT in mammals) which resides in the inner mitochondrial membrane and exchanges ADP and ATP to enable OXPHOS. Here, we investigated the role of these buried CLs in the carrier using yeast Aac2 as a model. We introduced negatively charged mutations into each CL-binding site of Aac2 to disrupt the CL interactions via electrostatic repulsion. While all mutations disturbing the CL-protein interaction destabilized Aac2 monomeric structure, transport activity was impaired in a pocket-specific manner. Finally, we determined that a disease-associated missense mutation in one CL-binding site in ANT1 compromised its structure and transport activity, resulting in OXPHOS defects. Our findings highlight the conserved significance of CL in AAC/ANT structure and function, directly tied to specific lipid-protein interactions.
Conflict of interest statement
Declaration of interests: The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
