Tumor-derived semaphorin 4A improves PD-1-blocking antibody efficacy by enhancing CD8+ T cell cytotoxicity and proliferation
- PMID: 37205755
- PMCID: PMC10198637
- DOI: 10.1126/sciadv.ade0718
Tumor-derived semaphorin 4A improves PD-1-blocking antibody efficacy by enhancing CD8+ T cell cytotoxicity and proliferation
Abstract
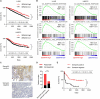
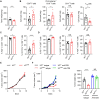
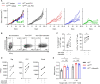
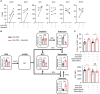
Immune checkpoint inhibitors (ICIs) have caused revolutionary changes in cancer treatment, but low response rates remain a challenge. Semaphorin 4A (Sema4A) modulates the immune system through multiple mechanisms in mice, although the role of human Sema4A in the tumor microenvironment remains unclear. This study demonstrates that histologically Sema4A-positive non-small cell lung cancer (NSCLC) responded significantly better to anti-programmed cell death 1 (PD-1) antibody than Sema4A-negative NSCLC. Intriguingly, SEMA4A expression in human NSCLC was mainly derived from tumor cells and was associated with T cell activation. Sema4A promoted cytotoxicity and proliferation of tumor-specific CD8+ T cells without terminal exhaustion by enhancing mammalian target of rapamycin complex 1 and polyamine synthesis, which led to improved efficacy of PD-1 inhibitors in murine models. Improved T cell activation by recombinant Sema4A was also confirmed using isolated tumor-infiltrating T cells from patients with cancer. Thus, Sema4A might be a promising therapeutic target and biomarker for predicting and promoting ICI efficacy.
Figures



References
-
- G. G. Lagos, B. Izar, N. A. Rizvi, Beyond tumor PD-L1: Emerging genomic biomarkers for checkpoint inhibitor immunotherapy. Am. Soc. Clin. Oncol. Educ. Book 40, 1–11 (2020). - PubMed
-
- S. H. Baumeister, G. J. Freeman, G. Dranoff, A. H. Sharpe, Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016). - PubMed
-
- P. Chongsathidkiet, C. Jackson, S. Koyama, F. Loebel, X. Cui, S. H. Farber, K. Woroniecka, A. A. Elsamadicy, C. A. Dechant, H. R. Kemeny, L. Sanchez-Perez, T. A. Cheema, N. C. Souders, J. E. Herndon, J. V. Coumans, J. I. Everitt, B. V. Nahed, J. H. Sampson, M. D. Gunn, R. L. Martuza, G. Dranoff, W. T. Curry, P. E. Fecci, Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 24, 1459–1468 (2018). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

