Dynamics of single cell femtosecond laser printing
- PMID: 37206114
- PMCID: PMC10191647
- DOI: 10.1364/BOE.480286
Dynamics of single cell femtosecond laser printing
Abstract
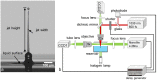
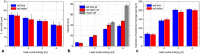
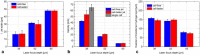
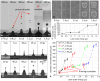
In the present study, we investigated the dynamics of a femtosecond (fs) laser induced bio-printing with cell-free and cell-laden jets under the variation of laser pulse energy and focus depth, by using time-resolved imaging. By increasing the laser pulse energy or decreasing the focus depth thresholds for a first and second jet are exceeded and more laser pulse energy is converted to kinetic jet energy. With increasing jet velocity, the jet behavior changes from a well-defined laminar jet, to a curved jet and further to an undesired splashing jet. We quantified the observed jet forms with the dimensionless hydrodynamic Weber and Rayleigh numbers and identified the Rayleigh breakup regime as the preferred process window for single cell bioprinting. Herein, the best spatial printing resolution of 42 ± 3 µm and single cell positioning precision of 12.4 µm are reached, which is less than one single cell diameter about 15 µm.
© 2023 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures




Similar articles
-
Effects of living cells on the bioink printability during laser printing.Biomicrofluidics. 2017 Jun 15;11(3):034120. doi: 10.1063/1.4985652. eCollection 2017 May. Biomicrofluidics. 2017. PMID: 28670353 Free PMC article.
-
Extending Single Cell Bioprinting from Femtosecond to Picosecond Laser Pulse Durations.Micromachines (Basel). 2021 Sep 29;12(10):1172. doi: 10.3390/mi12101172. Micromachines (Basel). 2021. PMID: 34683222 Free PMC article.
-
Time-Resolved Imaging Study of Jetting Dynamics during Laser Printing of Viscoelastic Alginate Solutions.Langmuir. 2015 Jun 16;31(23):6447-56. doi: 10.1021/acs.langmuir.5b00919. Epub 2015 Jun 4. Langmuir. 2015. PMID: 26011320
-
Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution.Biofabrication. 2014 Sep 12;6(4):045001. doi: 10.1088/1758-5082/6/4/045001. Biofabrication. 2014. PMID: 25215452
-
Study of Impingement Types and Printing Quality during Laser Printing of Viscoelastic Alginate Solutions.Langmuir. 2016 Mar 29;32(12):3004-14. doi: 10.1021/acs.langmuir.6b00220. Epub 2016 Mar 14. Langmuir. 2016. PMID: 26934283
Cited by
-
mSLAb - An open-source masked stereolithography (mSLA) bioprinter.HardwareX. 2024 Jun 10;19:e00543. doi: 10.1016/j.ohx.2024.e00543. eCollection 2024 Sep. HardwareX. 2024. PMID: 38988373 Free PMC article.
-
Time-resolved probing of laser-induced nanostructuring processes in liquids.Beilstein J Nanotechnol. 2025 Jul 2;16:968-1002. doi: 10.3762/bjnano.16.74. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 40625376 Free PMC article. Review.
References
-
- Duocastella M., “Laser-induced Forward Transfer of Liquids for Miniaturized Biosensors Preparation,” J. Laser Micro/Nanoeng. 3(1), 1–4 (2008).10.2961/jlmn.2008.01.0001 - DOI
-
- Serra P., “Laser-induced forward Transfer: a Direct-writing Technique for Biosensors Preparation,” J. Laser Micro/Nanoeng. 1(3), 236–242 (2006).10.2961/jlmn.2006.03.0017 - DOI
-
- Serra P., Colina M., Fernández-Pradas J. M., Sevilla L., Morenza J. L., “Preparation of functional DNA microarrays through laser-induced forward transfer,” Appl. Phys. Lett. 85(9), 1639–1641 (2004).10.1063/1.1787614 - DOI
LinkOut - more resources
Full Text Sources
