Signal attenuation-compensated projection-resolved OCT angiography
- PMID: 37206138
- PMCID: PMC10191650
- DOI: 10.1364/BOE.483835
Signal attenuation-compensated projection-resolved OCT angiography
Abstract
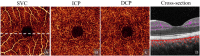
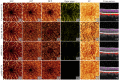
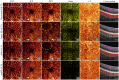
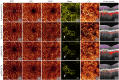
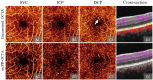
Projection artifacts are a significant limitation of optical coherence tomographic angiography (OCTA). Existing techniques to suppress these artifacts are sensitive to image quality, becoming less reliable on low-quality images. In this study, we propose a novel signal attenuation-compensated projection-resolved OCTA (sacPR-OCTA) algorithm. In addition to removing projection artifacts, our method compensates for shadows beneath large vessels. The proposed sacPR-OCTA algorithm improves vascular continuity, reduces the similarity of vascular patterns in different plexuses, and removes more residual artifacts compared to existing methods. In addition, the sacPR-OCTA algorithm better preserves flow signal in choroidal neovascular lesions and shadow-affected areas. Because sacPR-OCTA processes the data along normalized A-lines, it provides a general solution for removing projection artifacts agnostic to the platform.
© 2023 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
Jie Wang: Optovue/Visionix, Inc (P, R); David Huang: Optovue/Visionix, Inc. (F, P, R), Boeringer Ingelheim Inc. (C); Yali Jia: Optovue/Visionix, Inc. (P, R), Optos Inc. (P).
Figures



References
-
- Hwang T. S., Gao S. S., Liu L., Lauer A. K., Bailey S. T., Flaxel C. J., Wilson D. J., Huang D., Jia Y., “Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy,” JAMA Ophthalmol. 134(4), 367–373 (2016).10.1001/jamaophthalmol.2015.5658 - DOI - PMC - PubMed
-
- Zhang M., Hwang T. S., Dongye C., Wilson D. J., Huang D., Jia Y., “Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy,” Invest. Ophthalmol. Visual Sci. 57, 5101–5106 (2016).10.1167/iovs.16-19776 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
