Transcription activation by the resistance protein AlbA as a tool to evaluate derivatives of the antibiotic albicidin
- PMID: 37206387
- PMCID: PMC10189885
- DOI: 10.1039/d3sc00955f
Transcription activation by the resistance protein AlbA as a tool to evaluate derivatives of the antibiotic albicidin
Abstract
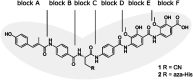
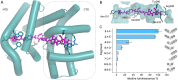
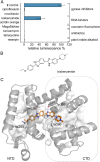
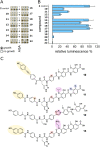
The rising numbers of fatal infections with resistant pathogens emphasizes the urgent need for new antibiotics. Ideally, new antibiotics should be able to evade or overcome existing resistance mechanisms. The peptide antibiotic albicidin is a highly potent antibacterial compound with a broad activity spectrum but also with several known resistance mechanisms. In order to assess the effectiveness of novel albicidin derivatives in the presence of the binding protein and transcription regulator AlbA, a resistance mechanism against albicidin identified in Klebsiella oxytoca, we designed a transcription reporter assay. In addition, by screening shorter albicidin fragments, as well as various DNA-binders and gyrase poisons, we were able to gain insights into the AlbA target spectrum. We analysed the effect of mutations in the binding domain of AlbA on albicidin sequestration and transcription activation, and found that the signal transduction mechanism is complex but can be evaded. Further demonstrating AlbA's high level of specificity, we find clues for the logical design of molecules capable of avoiding the resistance mechanism.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Murray C. J. Ikuta K. S. Sharara F. Swetschinski L. Robles Aguilar G. Gray A. Han C. Bisignano C. Rao P. Wool E. Johnson S. C. Browne A. J. Chipeta M. G. Fell F. Hackett S. Haines-Woodhouse G. Kashef Hamadani B. H. Kumaran E. A. P. McManigal B. Agarwal R. Akech S. Albertson S. Amuasi J. Andrews J. Aravkin A. Ashley E. Bailey F. Baker S. Basnyat B. Bekker A. Bender R. Bethou A. Bielicki J. Boonkasidecha S. Bukosia J. Carvalheiro C. Castañeda-Orjuela C. Chansamouth V. Chaurasia S. Chiurchiù S. Chowdhury F. Cook A. J. Cooper B. Cressey T. R. Criollo-Mora E. Cunningham M. Darboe S. Day N. P. J. De Luca M. Dokova K. Dramowski A. Dunachie S. J. Eckmanns T. Eibach D. Emami A. Feasey N. Fisher-Pearson N. Forrest K. Garrett D. Gastmeier P. Giref A. Z. Greer R. C. Gupta V. Haller S. Haselbeck A. Hay S. I. Holm M. Hopkins S. Iregbu K. C. Jacobs J. Jarovsky D. Javanmardi F. Khorana M. Kissoon N. Kobeissi E. Kostyanev T. Krapp F. Krumkamp R. Kumar A. Kyu H. H. Lim C. Limmathurotsakul D. Loftus M. J. Lunn M. Ma J. Mturi N. Munera-Huertas T. Musicha P. Mussi-Pinhata M. M. Nakamura T. Nanavati R. Nangia S. Newton P. Ngoun C. Novotney A. Nwakanma D. Obiero C. W. Olivas-Martinez A. Olliaro P. Ooko E. Ortiz-Brizuela E. Peleg A. Y. Perrone C. Plakkal N. Ponce-de-Leon A. Raad M. Ramdin T. Riddell A. Roberts T. Robotham J. V. Roca A. Rudd K. E. Russell N. Schnall J. Scott J. A. G. Shivamallappa M. Sifuentes-Osornio J. Steenkeste N. Stewardson A. J. Stoeva T. Tasak N. Thaiprakong A. Thwaites G. Turner C. Turner P. van Doorn H. R. Velaphi S. Vongpradith A. Vu H. Walsh T. Waner S. Wangrangsimakul T. Wozniak T. Zheng P. Sartorius B. Lopez A. D. Stergachis A. Moore C. Dolecek C. Naghavi M. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

