Expression of S100A9 in adamantinomatous craniopharyngioma and its association with wet keratin formation
- PMID: 37206553
- PMCID: PMC10189609
- DOI: 10.3892/etm.2023.11981
Expression of S100A9 in adamantinomatous craniopharyngioma and its association with wet keratin formation
Abstract
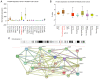
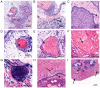
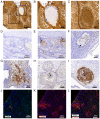
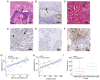
Wet keratin is a hallmark of adamantinomatous craniopharyngioma (ACP), which is frequently infiltrated by inflammatory cells. S100 calcium-binding protein A9 (S100A9) has been confirmed to play a decisive role in the development of inflammation. However, the relationship between wet keratin (keratin nodules) and S100A9 in ACP is poorly understood. The objective of the present study was to explore the expression of S100A9 in ACP and its association with wet keratin formation. Immunohistochemistry and immunofluorescence were used to detect the expression of S100A9, β-catenin and Ki67 in 46 cases of ACP. A total of three online databases were used to analyze S100A9 gene expression and protein data. The results revealed that S100A9 was primarily expressed in wet keratin and some intratumoral and peritumoral cells, and its expression in wet keratin was upregulated in the high inflammation group (P=1.800x10-3). In addition, S100A9 was correlated with the degree of inflammation (r=0.6; P=7.412x10-3) and the percentage of Ki67-positive cells (r=0.37; P=1.000x10-2). In addition, a significant correlation was noted between the area of wet keratin and the degree of inflammation (r=0.51; P=2.500x10-4). In conclusion, the present study showed that S100A9 was upregulated in ACP and may be closely associated with wet keratin formation and the infiltration of inflammatory cells in ACP.
Keywords: Ki67; S100 calcium-binding protein A9; adamantinomatous craniopharyngioma; inflammation; wet keratin.
Copyright: © Zhao et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
