Solar Photocatalytic Phenol Polymerization and Hydrogen Generation for Flocculation of Wastewater Impurities
- PMID: 37206614
- PMCID: PMC10194423
- DOI: 10.1021/acsapm.9b00210
Solar Photocatalytic Phenol Polymerization and Hydrogen Generation for Flocculation of Wastewater Impurities
Abstract
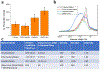
Achieving global sustainability will require balancing encroaching climate changes while maintaining existing quality of life. Using sunlight to purify wastewater while simultaneously generating usable fuels is an opportunity to approach both targets in a cost-efficient manner. In addition, converting biomass products to usable polymers is a sustainable approach for potentially replacing polystyrene or other petroleum derived polymers. Phenols from medical, manufacturing, and agricultural waste are commonly found in many water sources, and they are known to foul common reverse osmosis membranes. Here, we show oxidative polymerization of guaiacol, an aromatic compound derived from biomass, with concurrent hydrogen gas generation by using platinum-seeded cadmium sulfide nanorods (Pt@CdS) as photocatalysts. Rather than forming short oligomers as typically made by enzymes such as laccase and peroxidase, the resulting polymers show higher molecular weights that can more easily flocculate out of water. By comparing guaiacol conversion to molecular weight and dispersity, the guaiacol was found to polymerize via a chain-growth process. We also show that Pt@CdS can polymerize other phenols as well by testing the monomers phenol, 2,6-dihydroxybenzoic acid, gallic acid, and vanillin. Lastly, because the aqueous solubility of these aromatic polymers decreases dramatically with molecular weight, polymerization reactions were also tested in biphasic solutions to determine if chain growth could propagate in the oil phase. We show that the Pt@CdS nanoparticles can form stable Pickering emulsions in various biphasic combinations, and that both H2 formation and polymer molecular weight correlated with the partition coefficient of guaiacol into the oil phase as well as the solubility of the growing polymer chains. These combined studies demonstrate the possibility of using nanoscale photocatalysts to oxidatively polymerize phenolic substrates via a chain-growth mechanism, thereby providing a path for pretreating water by flocculating out contaminants with concurrent generation of hydrogen.
Keywords: Biphasic Systems; Green Chemistry; Phenol Polymerization; Photocatalysis; Wastewater purification.
Figures



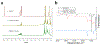
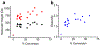
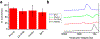
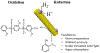
References
-
- Moniz EJ The Water-Energy Nexus: Challenges and Opportunities; U.S. Department of Energy, 2014.
-
- McCarty PL; Bae J; Kim J Domestic Wastewater Treatment as a Net Energy Producer–Can This Be Achieved? Environmental Science & Technology 2011, 45 (17), 7100–7106. - PubMed
-
- Heidrich ES; Curtis TP; Dolfing J Determination of the Internal Chemical Energy of Wastewater. Environmental Science & Technology 2011, 45 (2), 827–832. - PubMed
-
- Suss ME; Porada S; Sun X; Biesheuvel PM; Yoon J; Presser V Water Desalination via Capacitive Deionization: What Is It and What Can We Expect from It? Energy & Environmental Science 2015, 8 (8), 2296–2319.
-
- Ye M; Pasta M; Xie X; Cui Y; Criddle CS Performance of a Mixing Entropy Battery Alternately Flushed with Wastewater Effluent and Seawater for Recovery of Salinity-Gradient Energy. Energy Environ. Sci 2014, 7 (7), 2295–2300.
Grants and funding
LinkOut - more resources
Full Text Sources

