Novel targeted drugs for follicular and marginal zone lymphoma: a comprehensive review
- PMID: 37207160
- PMCID: PMC10189145
- DOI: 10.3389/fonc.2023.1170394
Novel targeted drugs for follicular and marginal zone lymphoma: a comprehensive review
Abstract
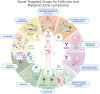
Although mostly incurable, indolent non-Hodgkin lymphomas (iNHL) are chronic diseases with a median overall survival approaching 20 years. In recent years, important advances in the knowledge of the biology of these lymphomas have led to the development of new drugs, mostly chemotherapy-free, with promising outcomes. With a median age of around 70 years at diagnosis, many patients with iNHL suffer from comorbid conditions that may limit treatment options. Therefore, nowadays, in the transition towards personalized medicine, several challenges lie ahead, such as identifying predictive markers for the selection of treatment, the adequate sequencing of available therapies, and the management of new and accumulated toxicities. In this review, we include a perspective on recent therapeutic advances in follicular and marginal zone lymphoma. We describe emerging data on approved and emerging novel therapies, such as targeted therapies (PI3K inhibitors, BTK inhibitors, EZH2 inhibitors), monoclonal antibodies and antibody-drug conjugates. Finally, we describe immune-directed approaches such as combinations with lenalidomide or the even more innovative bispecific T-cell engagers and chimeric antigen receptor T-cell therapy, which can achieve a high rate of durable responses with manageable toxicities, further obviating the need for chemotherapy.
Keywords: CAR-T cells; follicular lymphoma; immunotherapy; marginal zone lymphoma; targeted therapy.
Copyright © 2023 Rivero, Mozas, Magnano and López-Guillermo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program . Follicular lymphoma. Available at: https://seer.cancer.gov/seertools/hemelymph/51f6cf57e3e27c3994bd532a/ (Accessed February 19, 2023).
-
- Shi Q, Flowers CR, Hiddemann W, Marcus R, Herold M, Hagenbeek A, et al. . Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: an individual patient-level analysis of multiple randomized trials. J Clin Oncol (2017) 35(5):552–60. doi: 10.1200/JCO.2016.70.8651 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

