Brain effects of gestating germ-free persist in mouse neonates despite acquisition of a microbiota at birth
- PMID: 37207179
- PMCID: PMC10188942
- DOI: 10.3389/fnins.2023.1130347
Brain effects of gestating germ-free persist in mouse neonates despite acquisition of a microbiota at birth
Abstract
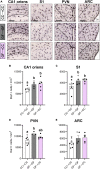
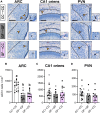
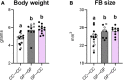
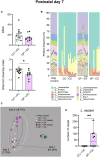
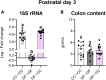
At birth, mammals experience a massive colonization by microorganisms. We previously reported that newborn mice gestated and born germ-free (GF) have increased microglial labeling and alterations in developmental neuronal cell death in the hippocampus and hypothalamus, as well as greater forebrain volume and body weight when compared to conventionally colonized (CC) mice. To test whether these effects are solely due to differences in postnatal microbial exposure, or instead may be programmed in utero, we cross-fostered GF newborns immediately after birth to CC dams (GF→CC) and compared them to offspring fostered within the same microbiota status (CC→CC, GF→GF). Because key developmental events (including microglial colonization and neuronal cell death) shape the brain during the first postnatal week, we collected brains on postnatal day (P) 7. To track gut bacterial colonization, colonic content was also collected and subjected to 16S rRNA qPCR and Illumina sequencing. In the brains of GF→GF mice, we replicated most of the effects seen previously in GF mice. Interestingly, the GF brain phenotype persisted in GF→CC offspring for almost all measures. In contrast, total bacterial load did not differ between the CC→CC and GF→CC groups on P7, and bacterial community composition was also very similar, with a few exceptions. Thus, GF→CC offspring had altered brain development during at least the first 7 days after birth despite a largely normal microbiota. This suggests that prenatal influences of gestating in an altered microbial environment programs neonatal brain development.
Keywords: bacterial load; cell death; colonic content; cross-fostering; forebrain size; microglia.
Copyright © 2023 Castillo-Ruiz, Gars, Sturgeon, Ronczkowski, Pyaram, Dauriat, Chassaing and Forger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

