Interleukin 36 receptor-inducible matrix metalloproteinase 13 mediates intestinal fibrosis
- PMID: 37207229
- PMCID: PMC10189878
- DOI: 10.3389/fimmu.2023.1163198
Interleukin 36 receptor-inducible matrix metalloproteinase 13 mediates intestinal fibrosis
Abstract
Background: Fibrostenotic disease is a common complication in Crohn's disease (CD) patients hallmarked by transmural extracellular matrix (ECM) accumulation in the intestinal wall. The prevention and medical therapy of fibrostenotic CD is an unmet high clinical need. Although targeting IL36R signaling is a promising therapy option, downstream mediators of IL36 during inflammation and fibrosis have been incompletely understood. Candidate molecules include matrix metalloproteinases which mediate ECM turnover and are thereby potential targets for anti-fibrotic treatment. Here, we have focused on understanding the role of MMP13 during intestinal fibrosis.
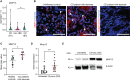
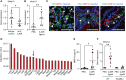
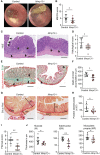
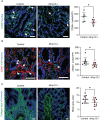
Methods: We performed bulk RNA sequencing of paired colon biopsies taken from non-stenotic and stenotic areas of patients with CD. Corresponding tissue samples from healthy controls and CD patients with stenosis were used for immunofluorescent (IF) staining. MMP13 gene expression was analyzed in cDNA of intestinal biopsies from healthy controls and in subpopulations of patients with CD in the IBDome cohort. In addition, gene regulation on RNA and protein level was studied in colon tissue and primary intestinal fibroblasts from mice upon IL36R activation or blockade. Finally, in vivo studies were performed with MMP13 deficient mice and littermate controls in an experimental model of intestinal fibrosis. Ex vivo tissue analysis included Masson's Trichrome and Sirius Red staining as well as evaluation of immune cells, fibroblasts and collagen VI by IF analysis.
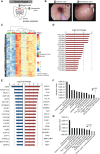
Results: Bulk RNA sequencing revealed high upregulation of MMP13 in colon biopsies from stenotic areas, as compared to non-stenotic regions of patients with CD. IF analysis confirmed higher levels of MMP13 in stenotic tissue sections of CD patients and demonstrated αSMA+ and Pdpn+ fibroblasts as a major source. Mechanistic experiments demonstrated that MMP13 expression was regulated by IL36R signaling. Finally, MMP13 deficient mice, as compared to littermate controls, developed less fibrosis in the chronic DSS model and showed reduced numbers of αSMA+ fibroblasts. These findings are consistent with a model suggesting a molecular axis involving IL36R activation in gut resident fibroblasts and MMP13 expression during the pathogenesis of intestinal fibrosis.
Conclusion: Targeting IL36R-inducible MMP13 could evolve as a promising approach to interfere with the development and progression of intestinal fibrosis.
Keywords: IL-36; collagen type VI; collagenase 3; extracellular matrix (ECM); il36 receptor; intestinal fibrosis; matrix metalloproteinase 13 (MMP13); αSMA+ fibroblasts.
Copyright © 2023 Koop, Enderle, Hillmann, Ruspeckhofer, Vieth, Sturm, Trajanoski, Kühl, Atreya, Leppkes, Baum, Roy, Martin, Neurath and Neufert.
Conflict of interest statement
Author PB was employed by Boehringer Ingelheim Pharma GmbH & Co KG. Author JR was employed by Staburo GmbH. Author AM was employed by Boehringer Ingelheim Pharmaceuticals Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

