Dysbiosis of gut microbiota inhibits NMNAT2 to promote neurobehavioral deficits and oxidative stress response in the 6-OHDA-lesioned rat model of Parkinson's disease
- PMID: 37208728
- PMCID: PMC10199500
- DOI: 10.1186/s12974-023-02782-1
Dysbiosis of gut microbiota inhibits NMNAT2 to promote neurobehavioral deficits and oxidative stress response in the 6-OHDA-lesioned rat model of Parkinson's disease
Abstract
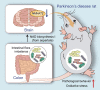
Background: New data are accumulating on gut microbial dysbiosis in Parkinson's disease (PD), while the specific mechanism remains uncharacterized. This study aims to investigate the potential role and pathophysiological mechanism of dysbiosis of gut microbiota in 6-hydroxydopamine (6-OHDA)-induced PD rat models.
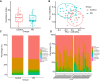
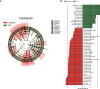
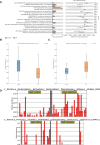
Methods: The shotgun metagenome sequencing data of fecal samples from PD patients and healthy individuals were obtained from the Sequence Read Archive (SRA) database. The diversity, abundance, and functional composition of gut microbiota were further analyzed in these data. After the exploration of the functional pathway-related genes, KEGG and GEO databases were used to obtain PD-related microarray datasets for differential expression analysis. Finally, in vivo experiments were performed to confirm the roles of fecal microbiota transplantation (FMT) and upregulated NMNAT2 in neurobehavioral symptoms and oxidative stress response in 6-OHDA-lesioned rats.
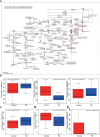
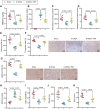
Results: Significant differences were found in the diversity, abundance, and functional composition of gut microbiota between PD patients and healthy individuals. Dysbiosis of gut microbiota could regulate NAD+ anabolic pathway to affect the occurrence and development of PD. As a NAD+ anabolic pathway-related gene, NMNAT2 was poorly expressed in the brain tissues of PD patients. More importantly, FMT or overexpression of NMNAT2 alleviated neurobehavioral deficits and reduced oxidative stress in 6-OHDA-lesioned rats.
Conclusions: Taken together, we demonstrated that dysbiosis of gut microbiota suppressed NMNAT2 expression, thus exacerbating neurobehavioral deficits and oxidative stress response in 6-OHDA-lesioned rats, which could be rescued by FMT or NMNAT2 restoration.
Keywords: Dysbiosis of gut microbiota; Fecal microbiota transplantation; NMNAT2; Neurobehavioral symptoms; Oxidative stress response; Parkinson’s disease.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

