Androgen receptor coordinates muscle metabolic and contractile functions
- PMID: 37208984
- PMCID: PMC10401547
- DOI: 10.1002/jcsm.13251
Androgen receptor coordinates muscle metabolic and contractile functions
Abstract
Background: Androgens are anabolic steroid hormones that exert their function by binding to the androgen receptor (AR). We have previously established that AR deficiency in limb muscles impairs sarcomere myofibrillar organization and decreases muscle strength in male mice. However, despite numerous studies performed in men and rodents, the signalling pathways controlled by androgens via their receptor in skeletal muscles remain poorly understood.
Methods: Male ARskm-/y (n = 7-12) and female ARskm-/- mice (n = 9), in which AR is selectively ablated in myofibres of musculoskeletal tissue, and male AR(i)skm-/y , in which AR is selectively ablated in post-mitotic skeletal muscle myofibres (n = 6), were generated. Longitudinal monitoring of body weight, blood glucose, insulin, lipids and lipoproteins was performed, alongside metabolomic analyses. Glucose metabolism was evaluated in C2C12 cells treated with 5α-dihydrotestosterone (DHT) and the anti-androgen flutamide (n = 6). Histological analyses on macroscopic and ultrastructural levels of longitudinal and transversal muscle sections were conducted. The transcriptome of gastrocnemius muscles from control and ARskm-/y mice was analysed at the age of 9 weeks (P < 0.05, 2138 differentially expressed genes) and validated by RT-qPCR analysis. The AR (4691 peaks with false discovery rate [FDR] < 0.1) and H3K4me2 (47 225 peaks with FDR < 0.05) cistromes in limb muscles were determined in 11-week-old wild-type mice.
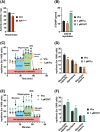
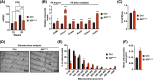
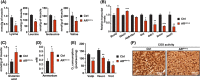
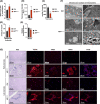
Results: We show that disrupting the androgen/AR axis impairs in vivo glycolytic activity and fastens the development of type 2 diabetes in male, but not in female mice. In agreement, treatment with DHT increases glycolysis in C2C12 myotubes by 30%, whereas flutamide has an opposite effect. Fatty acids are less efficiently metabolized in skeletal muscles of ARskm-/y mice and accumulate in cytoplasm, despite increased transcript levels of genes encoding key enzymes of beta-oxidation and mitochondrial content. Impaired glucose and fatty acid metabolism in AR-deficient muscle fibres is associated with 30% increased lysine and branched-chain amino acid catabolism, decreased polyamine biosynthesis and disrupted glutamate transamination. This metabolic switch generates ammonia (2-fold increase) and oxidative stress (30% increased H2 O2 levels), which impacts mitochondrial functions and causes necrosis in <1% fibres. We unravel that AR directly activates the transcription of genes involved in glycolysis, oxidative metabolism and muscle contraction.
Conclusions: Our study provides important insights into diseases caused by impaired AR function in musculoskeletal system and delivers a deeper understanding of skeletal muscle pathophysiological dynamics that is instrumental to develop effective treatment for muscle disorders.
Keywords: androgen receptor; genomics; metabolism; skeletal muscle; type 2 diabetes.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
None declared.
Figures




References
-
- Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 2010;199:451–463. - PubMed
-
- Handschin C, Chin S, Li P, Liu F, Maratos‐Flier E, LeBrasseur NK, et al. Skeletal muscle fiber‐type switching, exercise intolerance, and myopathy in PGC‐1α muscle‐specific knock‐out animals. J Biol Chem 2007;282:30014–30021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

