Synthesis and Evaluation of Diguanosine Cap Analogs Modified at the C8-Position by Suzuki-Miyaura Cross-Coupling: Discovery of 7-Methylguanosine-Based Molecular Rotors
- PMID: 37209102
- PMCID: PMC10242767
- DOI: 10.1021/acs.joc.3c00126
Synthesis and Evaluation of Diguanosine Cap Analogs Modified at the C8-Position by Suzuki-Miyaura Cross-Coupling: Discovery of 7-Methylguanosine-Based Molecular Rotors
Abstract
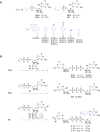
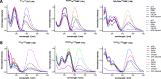
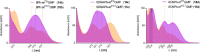
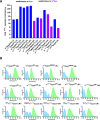
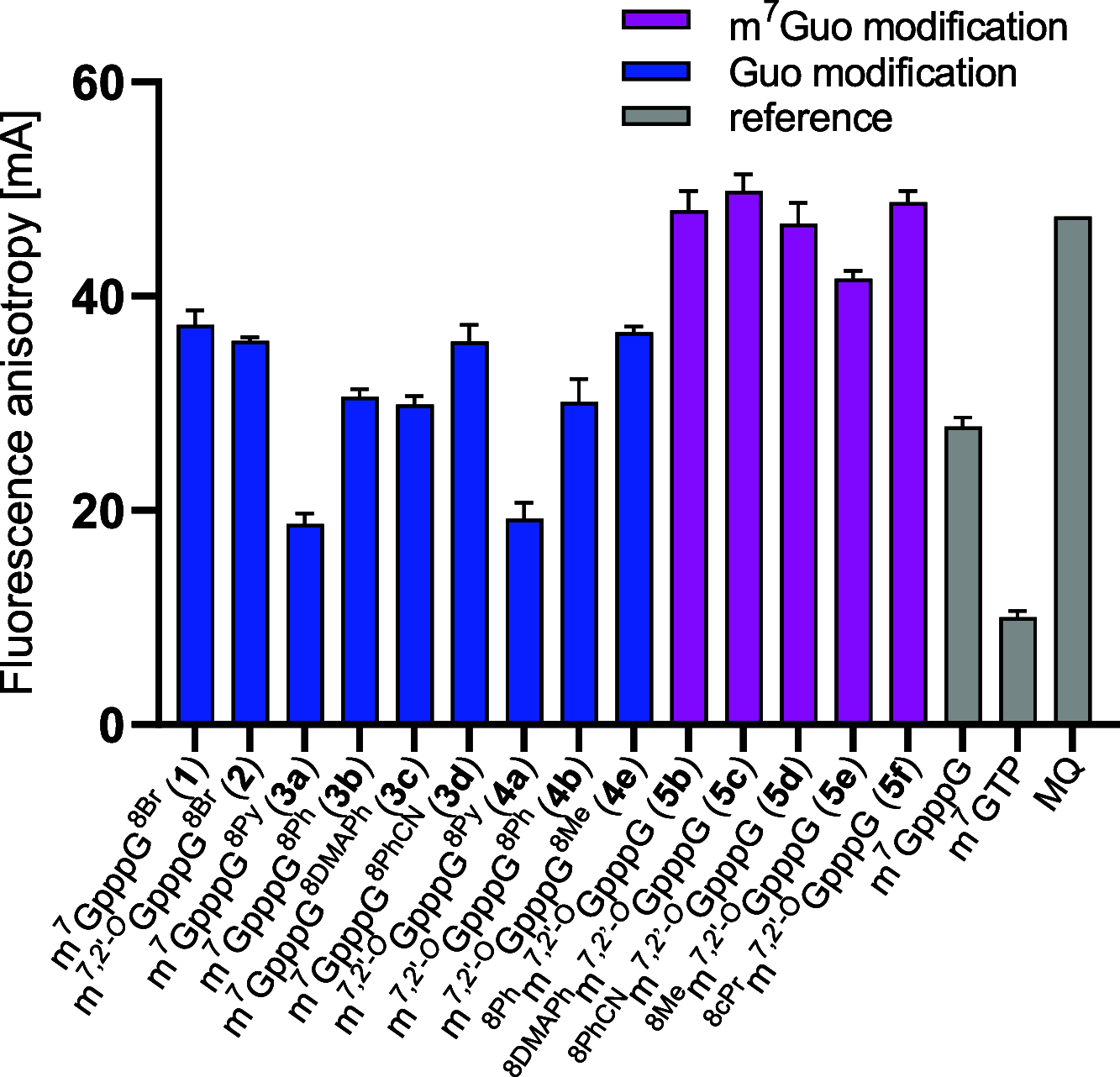
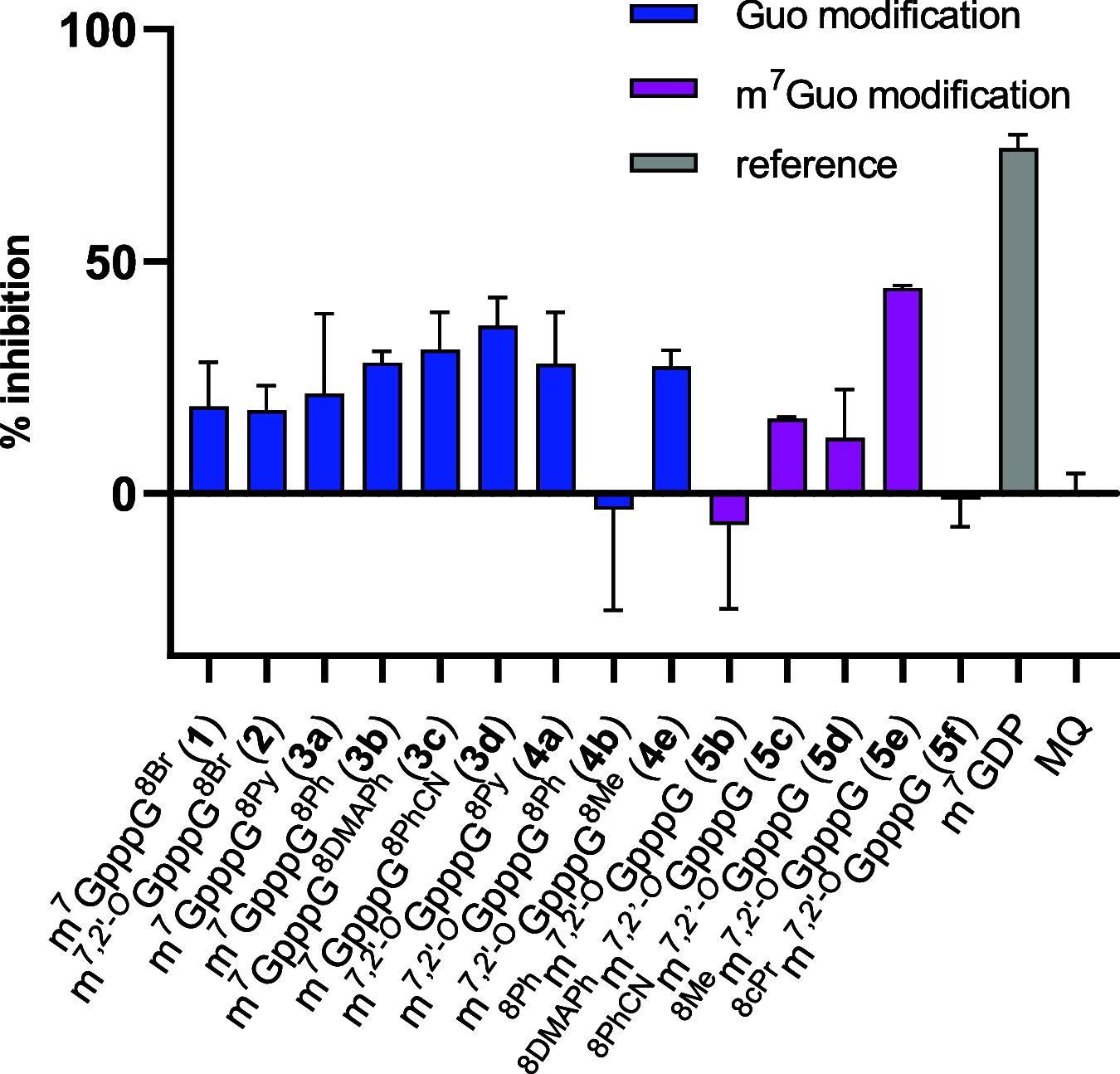
Chemical modifications of the mRNA cap structure can enhance the stability, translational properties, and half-life of mRNAs, thereby altering the therapeutic properties of synthetic mRNA. However, cap structure modification is challenging because of the instability of the 5'-5'-triphosphate bridge and N7-methylguanosine. The Suzuki-Miyaura cross-coupling reaction between boronic acid and halogen compound is a mild, convenient, and potentially applicable approach for modifying biomolecules. Herein, we describe two methods to synthesize C8-modified cap structures using the Suzuki-Miyaura cross-coupling reaction. Both methods employed phosphorimidazolide chemistry to form the 5',5'-triphosphate bridge. However, in the first method, the introduction of the modification via the Suzuki-Miyaura cross-coupling reaction at the C8 position occurs postsynthetically, at the dinucleotide level, whereas in the second method, the modification was introduced at the level of the nucleoside 5'-monophosphate, and later, the triphosphate bridge was formed. Both methods were successfully applied to incorporate six different groups (methyl, cyclopropyl, phenyl, 4-dimethylaminophenyl, 4-cyanophenyl, and 1-pyrene) into either the m7G or G moieties of the cap structure. Aromatic substituents at the C8-position of guanosine form a push-pull system that exhibits environment-sensitive fluorescence. We demonstrated that this phenomenon can be harnessed to study the interaction with cap-binding proteins, e.g., eIF4E, DcpS, Nudt16, and snurportin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Novel N7-Arylmethyl Substituted Dinucleotide mRNA 5' cap Analogs: Synthesis and Evaluation as Modulators of Translation.Pharmaceutics. 2021 Nov 16;13(11):1941. doi: 10.3390/pharmaceutics13111941. Pharmaceutics. 2021. PMID: 34834356 Free PMC article.
-
Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS.RNA. 2008 Jun;14(6):1119-31. doi: 10.1261/rna.990208. Epub 2008 Apr 22. RNA. 2008. PMID: 18430890 Free PMC article.
-
N1-Propargylguanosine Modified mRNA Cap Analogs: Synthesis, Reactivity, and Applications to the Study of Cap-Binding Proteins.Molecules. 2019 May 17;24(10):1899. doi: 10.3390/molecules24101899. Molecules. 2019. PMID: 31108861 Free PMC article.
-
Applications of Phosphate Modification and Labeling to Study (m)RNA Caps.Top Curr Chem (Cham). 2017 Feb;375(1):16. doi: 10.1007/s41061-017-0106-y. Epub 2017 Jan 23. Top Curr Chem (Cham). 2017. PMID: 28116583 Free PMC article. Review.
-
Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability.Methods Enzymol. 2007;431:203-27. doi: 10.1016/S0076-6879(07)31011-2. Methods Enzymol. 2007. PMID: 17923237 Review.
Cited by
-
Stereoselective chemoenzymatic phytate transformations provide access to diverse inositol phosphate derivatives.Chem Sci. 2025 Jun 23;16(29):13459-13467. doi: 10.1039/d5sc02844b. eCollection 2025 Jul 23. Chem Sci. 2025. PMID: 40584237 Free PMC article.
-
Physicochemical and functional assessment of messenger RNA 5'Cap-end impurities under forced degradation conditions.Mol Ther Nucleic Acids. 2025 May 20;36(2):102570. doi: 10.1016/j.omtn.2025.102570. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40520363 Free PMC article.
-
Application of Mammalian Nudix Enzymes to Capped RNA Analysis.Pharmaceuticals (Basel). 2024 Sep 11;17(9):1195. doi: 10.3390/ph17091195. Pharmaceuticals (Basel). 2024. PMID: 39338357 Free PMC article. Review.
References
-
- Sonenberg N.; Rupprecht K. M.; Hecht S. M.; Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 4345–4349. 10.1073/pnas.76.9.4345. - DOI - PMC - PubMed
- Mitchell S. F.; Walker S. E.; Algire M. A.; Park E. H.; Hinnebusch A. G.; Lorsch J. R. The 5′-7-methylguanosine cap on eukaryotic mRNAs serves both to stimulate canonical translation initiation and to block an alternative pathway. Mol. Cell 2010, 39, 950–962. 10.1016/j.molcel.2010.08.021. - DOI - PMC - PubMed
-
- Miyagi Y.; Sugiyama A.; Asai A.; Okazaki T.; Kuchino Y.; Kerr S. J. Elevated levels of eukaryotic translation initiation factor eIF-4E, mRNA in a broad-spectrum of transformed-cell lines. Cancer Lett. 1995, 91, 247–252. 10.1016/0304-3835(95)03737-h. - DOI - PubMed
- De Benedetti A.; Harris A. L. eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell Biol. 1999, 31, 59–72. 10.1016/s1357-2725(98)00132-0. - DOI - PubMed
-
- Singh J.; Salcius M.; Liu S. W.; Staker B. L.; Mishra R.; Thurmond J.; Michaud G.; Mattoon D. R.; Printen J.; Christensen J.; Bjornsson J. M.; Pollok B. A.; Kiledjian M.; Stewart L.; Jarecki J.; Gurney M. E. DcpS as a therapeutic target for spinal muscular atrophy. ACS Chem. Biol. 2008, 3, 711–722. 10.1021/cb800120t. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

