Sequential CAR T cell and targeted alpha immunotherapy in disseminated multiple myeloma
- PMID: 37209218
- PMCID: PMC10361855
- DOI: 10.1007/s00262-023-03461-z
Sequential CAR T cell and targeted alpha immunotherapy in disseminated multiple myeloma
Abstract
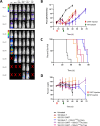
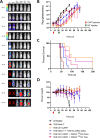
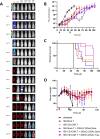
Multiple myeloma (MM) is still an incurable disorder despite improved antibody and cellular therapies against different MM antigens. Single targeted antigens have so far been ineffective against MM with most patients relapsing after initial response. Hence, sequential immunotherapies directed at different targets are expected to perform better than monotherapy alone. Here, we optimized and established in preclinical studies the therapeutic rationale of using targeted alpha therapy (TAT) directed against CD38 antigen (225Ac-DOTA-daratumumab) with CAR T cell therapy directed at CS1 antigen in a systemic MM model. The sequential therapies compared CAR T therapy followed by TAT to TAT followed by CAR T therapy. CAR T cell monotherapy increased median survival from 49 days (d) in untreated controls to 71d with a modest improvement to 89d for 3.7 kBq of TAT given 14d later. When CAR T was followed by 7.4 kBq of TAT 29d later, sequential therapy increased median survival from 47d in untreated controls to 106d, compared to 68d for CAR T monotherapy. When CAR T therapy was followed by untargeted alpha immunotherapy using 7.4 kBq of 225Ac-DOTA-trastuzumab (anti-HER2) antibody 29d later, there was only a slight improvement in response over CAR T monotherapy demonstrating the role of tumor targeting. TAT (7.4 kBq) followed by CAR T therapy was also effective when CAR T therapy was delayed for 21d vs 14d or 28d post TAT, highlighting the importance of timing sequential therapies. Sequential targeted therapies using CS1 CAR T or 225Ac-DOTA-CD38 TAT in either order shows promise over monotherapies alone.
Keywords: CAR T therapy; Multiple myeloma; Targeted alpha therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER cancer statistics review, National Cancer Institute, Bethesda, MD. 1975–2018
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

