Functional and Clinical Proteomic Exploration of Pancreatic Cancer
- PMID: 37209817
- PMCID: PMC10388587
- DOI: 10.1016/j.mcpro.2023.100575
Functional and Clinical Proteomic Exploration of Pancreatic Cancer
Abstract
Pancreatic cancer, in most cases being pancreatic ductal adenocarcinoma (PDAC), is one of the most lethal cancers with a median survival time of less than 6 months. Therapeutic options are very limited for patients with PDAC, and surgery is still the most effective treatment, making improvements in early diagnosis critical. One typical characteristic of PDAC is the desmoplastic reaction of its stroma microenvironment, which actively interacts with cancer cells to orchestrate key components in tumorigenesis, metastasis, and chemoresistance. A global exploration of cancer-stroma crosstalk is essential to decipher PDAC biology and design intervention strategies. Over the past decade, the dramatic improvement in proteomics technologies has enabled the profiling of proteins, post-translational modifications (PTMs), and their protein complexes at unprecedented sensitivity and dimensionality. Here, starting with our current understanding of PDAC characteristics, including precursor lesions, progression models, tumor microenvironment, and therapeutic advancements, we describe how proteomics contributes to the functional and clinical exploration of PDAC, providing insights into PDAC carcinogenesis, progression, and chemoresistance. We summarize recent achievements enabled by proteomics to systematically investigate PTMs-mediated intracellular signaling in PDAC, cancer-stroma interactions, and potential therapeutic targets revealed by these functional studies. We also highlight proteomic profiling of clinical tissue and plasma samples to discover and verify useful biomarkers that can aid early detection and molecular classification of patients. In addition, we introduce spatial proteomic technology and its applications in PDAC for deconvolving tumor heterogeneity. Finally, we discuss future prospects of applying new proteomic technologies in comprehensively understanding PDAC heterogeneity and intercellular signaling networks. Importantly, we expect advances in clinical functional proteomics for exploring mechanisms of cancer biology directly by high-sensitivity functional proteomic approaches starting from clinical samples.
Keywords: cancer-stroma crosstalk; clinical proteomics; exosome; functional proteomics; pancreatic cancer; post-translational modifications; spatial proteomics; tumor microenvironment.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

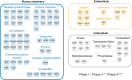
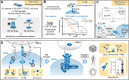

References
-
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. - PubMed
-
- Kleeff J., Korc M., Apte M., La Vecchia C., Johnson C.D., Biankin A.V., et al. Pancreatic cancer. Nat. Rev. Dis. Primers. 2016;2 - PubMed
-
- Nevala-Plagemann C., Hidalgo M., Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 2020;17:108–123. - PubMed
-
- Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379:2395–2406. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

