VACCELERATE Site Network: Real-time definition of clinical study capacity in Europe
- PMID: 37210309
- PMCID: PMC10194816
- DOI: 10.1016/j.vaccine.2023.05.006
VACCELERATE Site Network: Real-time definition of clinical study capacity in Europe
Abstract
Background: The inconsistent European vaccine trial landscape rendered the continent of limited interest for vaccine developers. The VACCELERATE consortium created a network of capable clinical trial sites throughout Europe. VACCELERATE identifies and provides access to state-of-the-art vaccine trial sites to accelerate clinical development of vaccines.
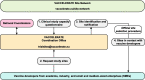
Methods: Login details for the VACCELERATE Site Network (vaccelerate.eu/site-network/) questionnaire can be obtained after sending an email to. Interested sites provide basic information, such as contact details, affiliation with infectious disease networks, main area of expertise, previous vaccine trial experience, site infrastructure and preferred vaccine trial settings. In addition, sites can recommend other clinical researchers for registration in the network. If directly requested by a sponsor or sponsor representative, the VACCELERATE Site Network pre-selects vaccine trial sites and shares basic study characteristics provided by the sponsor. Interested sites provide feedback with short surveys and feasibility questionnaires developed by VACCELERATE and are connected with the sponsor to initiate the site selection process.
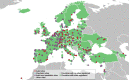
Results: As of April 2023, 481 sites from 39 European countries have registered in the VACCELERATE Site Network. Of these, 137 (28.5 %) sites have previous experience conducting phase I trials, 259 (53.8 %) with phase II, 340 (70.7 %) with phase III, and 205 (42.6 %) with phase IV trials, respectively. Infectious diseases were reported as main area of expertise by 274 sites (57.0 %), followed by any kind of immunosuppression by 141 (29.3 %) sites. Numbers are super additive as sites may report clinical trial experience in several indications. Two hundred and thirty-one (47.0 %) sites have the expertise and capacity to enrol paediatric populations and 391 (79.6 %) adult populations. Since its launch in October 2020, the VACCELERATE Site Network has been used 21 times for academic and industry trials, mostly interventional studies, focusing on different pathogens such as fungi, monkeypox virus, Orthomyxoviridae/influenza viruses, SARS-CoV-2, or Streptococcus pneumoniae/pneumococcus.
Conclusions: The VACCELERATE Site Network enables a constantly updated Europe-wide mapping of experienced clinical sites interested in executing vaccine trials. The network is already in use as a rapid-turnaround single contact point for the identification of vaccine trials sites in Europe.
Keywords: Clinical network; Pandemic preparedness; Registry; SARS-CoV-2; Site; Vaccine trial.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: VACCELERATE Consortium reports financial support was provided by European Union. VACCELERATE Consortium reports financial support was provided by Federal Ministry of Education and Research Bonn Office.
Figures
References
-
- Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368 - PubMed
-
- Centers for Disease Control and Prevention. Vaccine Testing and the Approval Process www.cdc.gov/vaccines/basics/test-approve.html (Last accessed August 3, 2022).
-
- European Vaccination Information Portal. Approval of vaccines in the European Union www.vaccination-info.eu/en/vaccine-facts/approval-vaccines-european-union (Last accessed August 2, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

