Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV
- PMID: 37210393
- PMCID: PMC10199661
- DOI: 10.1038/s41598-023-35328-9
Heterologous vaccination utilizing viral vector and protein platforms confers complete protection against SFTSV
Abstract
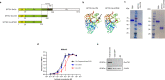
Severe fever with thrombocytopenia syndrome virus was first discovered in 2009 as the causative agent of severe fever with thrombocytopenia syndrome. Despite its potential threat to public health, no prophylactic vaccine is yet available. This study developed a heterologous prime-boost strategy comprising priming with recombinant replication-deficient human adenovirus type 5 (rAd5) expressing the surface glycoprotein, Gn, and boosting with Gn protein. This vaccination regimen induced balanced Th1/Th2 immune responses and resulted in potent humoral and T cell-mediated responses in mice. It elicited high neutralizing antibody titers in both mice and non-human primates. Transcriptome analysis revealed that rAd5 and Gn proteins induced adaptive and innate immune pathways, respectively. This study provides immunological and mechanistic insight into this heterologous regimen and paves the way for future strategies against emerging infectious diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Heterologous prime-boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus.Vaccine. 2018 Jun 7;36(24):3468-3476. doi: 10.1016/j.vaccine.2018.04.082. Epub 2018 May 5. Vaccine. 2018. PMID: 29739720 Free PMC article.
-
A rabies virus vectored severe fever with thrombocytopenia syndrome (SFTS) bivalent candidate vaccine confers protective immune responses in mice.Vet Microbiol. 2021 Jun;257:109076. doi: 10.1016/j.vetmic.2021.109076. Epub 2021 Apr 21. Vet Microbiol. 2021. PMID: 33957572
-
Prime-boost immunization using alphavirus replicon and adenovirus vectored vaccines induces enhanced immune responses against classical swine fever virus in mice.Vet Immunol Immunopathol. 2009 Oct 15;131(3-4):158-66. doi: 10.1016/j.vetimm.2009.04.003. Epub 2009 Apr 11. Vet Immunol Immunopathol. 2009. PMID: 19411115
-
Development of a candidate vaccine against severe fever with thrombocytopenia syndrome virus using Gn/Gc glycoprotein via multiple expression vectors delivered by attenuated Salmonella confers effective protection in hDC-SIGN transduced mice.Vaccine. 2025 Jan 1;43(Pt 1):126524. doi: 10.1016/j.vaccine.2024.126524. Epub 2024 Nov 14. Vaccine. 2025. PMID: 39547019
-
Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1.Expert Rev Vaccines. 2010 Sep;9(9):1055-69. doi: 10.1586/erv.10.106. Expert Rev Vaccines. 2010. PMID: 20822348 Review.
Cited by
-
Current Progress of Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) Vaccine Development.Viruses. 2024 Jan 16;16(1):128. doi: 10.3390/v16010128. Viruses. 2024. PMID: 38257828 Free PMC article. Review.
-
Recent research advances in the development of Dabie Banda virus vaccines.PLoS Negl Trop Dis. 2024 Aug 29;18(8):e0012411. doi: 10.1371/journal.pntd.0012411. eCollection 2024 Aug. PLoS Negl Trop Dis. 2024. PMID: 39207951 Free PMC article. Review.
-
Humoral immunity to phlebovirus infection.Ann N Y Acad Sci. 2023 Dec;1530(1):23-31. doi: 10.1111/nyas.15080. Epub 2023 Nov 7. Ann N Y Acad Sci. 2023. PMID: 37936483 Free PMC article. Review.
-
The Adaptive Immune Response against Bunyavirales.Viruses. 2024 Mar 21;16(3):483. doi: 10.3390/v16030483. Viruses. 2024. PMID: 38543848 Free PMC article. Review.
-
Safety, Immunogenicity, and Efficacy of a Recombinant Vesicular Stomatitis Virus Vectored Vaccine Against Severe Fever with Thrombocytopenia Syndrome Virus and Heartland Bandavirus.Vaccines (Basel). 2024 Dec 12;12(12):1403. doi: 10.3390/vaccines12121403. Vaccines (Basel). 2024. PMID: 39772063 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

