Origanum majorana L. protects against neuroinflammation-mediated cognitive impairment: a phyto-pharmacological study
- PMID: 37210483
- PMCID: PMC10199469
- DOI: 10.1186/s12906-023-03994-x
Origanum majorana L. protects against neuroinflammation-mediated cognitive impairment: a phyto-pharmacological study
Abstract
Background: Neuroinflammation and oxidative stress are critical players in the pathogenesis of numerous neurodegenerative diseases, such as Alzheimer's disease (AD) which is responsible for most cases of dementia in the elderly. With the lack of curative treatments, natural phenolics are potential candidates to delay the onset and progression of such age-related disorders due to their potent antioxidant and anti-inflammatory effects. This study aims at assessing the phytochemical characteristics of Origanum majorana L. (OM) hydroalcohol extract and its neuroprotective activities in a murine neuroinflammatory model.
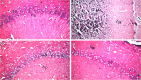
Methods: OM phytochemical analysis was done by HPLC/PDA/ESI-MSn. Oxidative stress was induced in vitro by hydrogen peroxide and cell viability was measured using WST-1 assay. Swiss albino mice were injected intraperitoneally with OM extract at a dose of 100 mg/kg for 12 days and with 250 μg/kg LPS daily starting from day 6 to induce neuroinflammation. Cognitive functions were assessed by novel object recognition and Y-maze behavioral tests. Hematoxylin and eosin staining was used to assess the degree of neurodegeneration in the brain. Reactive astrogliosis and inflammation were assessed by immunohistochemistry using GFAP and COX-2 antibodies, respectively.
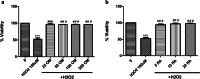
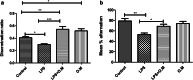
Results: OM is rich in phenolics, with rosmarinic acid and its derivatives being major constituents. OM extract and rosmarinic acid significantly protected microglial cells against oxidative stress-induced cell death (p < 0.001). OM protected against the LPS-induced alteration of recognition and spatial memory in mice (p < 0.001) and (p < 0.05), respectively. Mice that received OM extract prior to the induction of neuroinflammation showed comparable histology to control brains, with no overt neurodegeneration. Furthermore, OM pre-treatment decreased the immunohistochemistry profiler score of GFAP from positive to low positive and COX-2 from low positive to negative in the brain tissue, compared to the LPS group.
Conclusion: These findings highlight the potential preventive effects of OM phenolics against neuroinflammation and pave the way toward drug discovery and development for neurodegenerative disorders.
Keywords: Cognition; LPS; Neurodegeneration; Neuroinflammation; Origanum majorana; Phenolics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

