EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer
- PMID: 37210576
- PMCID: PMC10199584
- DOI: 10.1186/s12943-023-01786-y
EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer
Abstract
Background: Enhancer of zeste homolog 2 (EZH2), the key catalytic subunit of polycomb repressive complex 2 (PRC2), is overexpressed and plays an oncogenic role in various cancers through catalysis-dependent or catalysis-independent pathways. However, the related mechanisms contributing to ovarian cancer (OC) are not well understood.
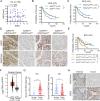
Methods: The levels of EZH2 and H3K27me3 were evaluated in 105 OC patients by immunohistochemistry (IHC) staining, and these patients were stratified based on these levels. Canonical and noncanonical binding sites of EZH2 were defined by chromatin immunoprecipitation sequencing (ChIP-Seq). The EZH2 solo targets were obtained by integrative analysis of ChIP-Seq and RNA sequencing data. In vitro and in vivo experiments were performed to determine the role of EZH2 in OC growth.
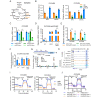
Results: We showed that a subgroup of OC patients with high EZH2 expression but low H3K27me3 exhibited the worst prognosis, with limited therapeutic options. We demonstrated that induction of EZH2 degradation but not catalytic inhibition profoundly blocked OC cell proliferation and tumorigenicity in vitro and in vivo. Integrative analysis of genome-wide chromatin and transcriptome profiles revealed extensive EZH2 occupancy not only at genomic loci marked by H3K27me3 but also at promoters independent of PRC2, indicating a noncanonical role of EZH2 in OC. Mechanistically, EZH2 transcriptionally upregulated IDH2 to potentiate metabolic rewiring by enhancing tricarboxylic acid cycle (TCA cycle) activity, which contributed to the growth of OC.
Conclusions: These data reveal a novel oncogenic role of EZH2 in OC and identify potential therapeutic strategies for OC by targeting the noncatalytic activity of EZH2.
Keywords: EZH2; IDH2; Metabolic rewiring; Ovarian cancer; TCA cycle.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

