Long-Term Postsurgical Outcomes of Neoadjuvant Chemoradiation (CROSS) Versus Chemotherapy (FLOT) for Multimodal Treatment of Adenocarcinoma of the Esophagus and the Esophagogastric Junction
- PMID: 37210683
- PMCID: PMC10562333
- DOI: 10.1245/s10434-023-13643-9
Long-Term Postsurgical Outcomes of Neoadjuvant Chemoradiation (CROSS) Versus Chemotherapy (FLOT) for Multimodal Treatment of Adenocarcinoma of the Esophagus and the Esophagogastric Junction
Abstract
Background: The question of the ideal neoadjuvant therapy for locally advanced esophagogastric adenocarcinoma has not been answered to date. Multimodal treatment has become a standard treatment for these adenocarcinomas. Currently, perioperative chemotherapy (FLOT) or neoadjuvant chemoradiation (CROSS) is recommended.
Methods: A monocentric retrospective analysis compared long-term survival after CROSS versus FLOT. The study enrolled patients with adenocarcinoma of the esophagus (EAC) or the esophagogastric junction type I or II undergoing oncologic Ivor-Lewis esophagectomy between January 2012 and December 2019. The primary objective was to determine the long-term outcome in terms of overall survival. The secondary objectives were to determine differences regarding the histopathologic categories after neoadjuvant treatment and the histomorphologic regression.
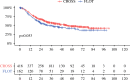
Results: The findings showed no survival advantage for one or the other treatment in this highly standardized cohort. All the patients underwent open (CROSS: 9.4% vs. FLOT: 22%), hybrid (CROSS: 82% vs. FLOT: 72%), or minimally invasive (CROSS: 8.9% vs. FLOT: 5.6%) thoracoabdominal esophagectomy. The median post-surgical follow-up period was 57.6 months (95% confidence interval [CI] 23.2-109.7 months), and the median survival was longer for the CROSS patients (54 months) than for the FLOT patients (37.2 months) (p = 0.053). The overall 5-years survival was 47% for the entire cohort (48% for the CROSS and 43% for the FLOT patients). The CROSS patients showed a better pathologic response and fewer advanced tumor stages.
Conclusion: The improved pathologic response after CROSS cannot be translated into longer overall survival. To date, the choice of which neoadjuvant treatment to use can be made only on the basis of clinical parameters and the patient's performance status.
Keywords: CROSS; Esophageal adenocarcinoma; FLOT; Long-term prognosis; Multimodal treatment; Neoadjuvant radiochemotherapy; Perioperative chemotherapy.
© 2023. The Author(s).
Conflict of interest statement
Hans Fuchs has an educational grant from the Intuitive Surgical | ESOMAP trial, serves on the advisory board of Activ Surgical, Medtronic, Stryker, and Distal Motion, and has stock options from Fortimedix Surgical. The remaining authors have no conflicts of interest.
Figures



Comment in
-
Customizing Therapy for Esophageal Cancer: CROSS vs. FLOT.Ann Surg Oncol. 2024 Jan;31(1):21-22. doi: 10.1245/s10434-023-14320-7. Epub 2023 Oct 13. Ann Surg Oncol. 2024. PMID: 37833462 No abstract available.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

